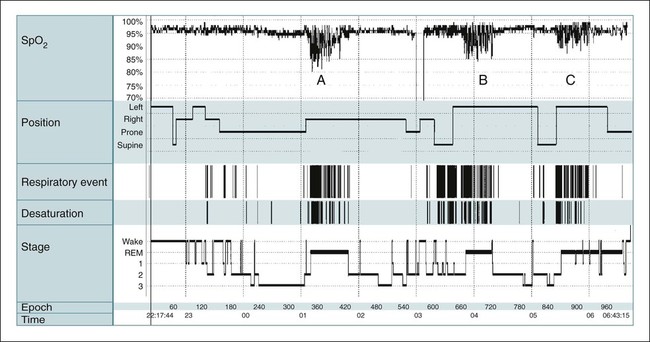
• PSG is indicated for evaluation of suspected OSA and for PAP titration. PSG is indicated for preoperative evaluation for planned surgery (to treat OSA or snoring), and postoperatively to document surgical effectiveness. • PSG is indicated to document effectiveness of oral appliance treatment of OSA. The practice parameters for oral appliance therapy state that a diagnostic study should be performed if an oral appliance is planned for snoring or suspected OSA. • PSG is indicated for evaluation of OSA patients being treated with CPAP or an oral appliance in whom symptoms have returned (PSG using CPAP or OA). • PSG is indicated if there is a clinical suspicion of OSA in patients with heart failure, coronary artery disease, recent or past stroke or transient ischemic attack, and neuromuscular disorders. • A repeat PSG is indicated if an initial study is negative but there is a high index of suspicion for OSA. • A PSG is indicated if there is weight loss (>10%) of an OSA patient on CPAP to determine whether CPAP is still indicated. • A PSG is indicated in a patient using CPAP after weight gain (>10%) to determine whether higher pressure is needed. • A PSG is NOT indicated for evaluation of the restless legs syndrome, insomnia, uncomplicated parasomnias, COPD, or a patient doing well on CPAP treatment. • Attended PM can be used as an alternative to PSG to diagnose OSA when there is a high pretest probability and to determine the effectiveness of therapy. Attended PM (type 3) is indicated if treatment is urgent and PSG is delayed or the patient cannot have a PSG in the sleep center owing to immobility or safety issues. • An unattended PM (type 3) is indicated to diagnose OSA in patients with a high probability of having moderate to severe OSA—IF combined with a comprehensive sleep evaluation and significant co-morbid medical disorders or sleep disorders that would benefit from PSG are excluded. A negative PM study should be followed by a PSG in most cases (to be studied by PM, there must be a high probability of having moderate to severe OSA). • Review of the raw PM data is essential to verify adequate technical quality of a study and to determine whether the respiratory events were properly scored. • The AASM PMGs recommended at a minimum airflow, respiratory effort, and oxygen saturation should be monitored. • Qualified providers should either place the PM sensors or instruct the patients to avoid a high proportion of technically inadequate studies. • Selection of the PM device should take into account the setting in which it will be used and whether the patient will place the sensors without assistance at home. The American Academy of Sleep Medicine (AASM) practice parameters1–6 outline the indications for polysomnography (PSG) (Tables 13–1 to 13–4 and Appendix 13–1). PSG is the standard test for diagnosis of suspected sleep-related breathing disorders (SRBDs), narcolepsy (when combined with a multiple sleep latency test [MSLT]), positive airway pressure (PAP) titration, and evaluation of parasomnias (under certain conditions).2,6 PSG is also indicated to document the efficacy of prior surgical treatment and determine the efficacy of oral appliance (OA) treatment (PSG while wearing the OA).2,6 The 2005 practice parameters for PSG stated that attended cardiorespiratory pulmonary studies (airflow + effort or two effort channels, oximetry, and electrocardiogram [ECG] or heart rate) are an acceptable alternative to PSG for diagnosis of obstructive sleep apnea (OSA) in patients with high pretest probability of OSA2,7 or for determining the adequacy of prior surgery for OSA or effectiveness of current OA treatment.2,6,7 In 2007, the AASM published Clinical Guidelines for the Use of Portable Monitoring in Evaluation of Adult Patients with OSA8 (hereafter referred to as PMGs). The PMGs state that unattended portable monitoring (PM) “may be” acceptable to monitor patients with a high probability of having moderate to severe OSA, if monitoring is performed according to the guidelines. Unattended PM monitoring is also acceptable for assessing the efficacy of non-PAP treatment of OSA. The Centers for Medicare and Medicaid Services (CMS) now recognize a diagnosis of OSA by unattended PM as acceptable for reimbursement of continuous positive airway pressure (CPAP) treatment9 and considers unattended PM studies reimbursable provided certain conditions are met.10 TABLE 13–1 Indications for Polysomnography (Sleep-Related Breathing Disorders) *Amended by practice parameters for oral appliance treatment to include all severities of OSA.6 TABLE 13–2 Indications for Polysomnography (Positive Airway Pressure Titration and Treatment) AHI = number of apneas + hypopneas/hr of sleep. From Kushida CA, Morgenthaler T, Littner MR, et al: Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006;29:240–243. TABLE 13–3 Indications for Polysomnography in Nonrespiratory Sleep Disorders • Nocturnal seizure is suspected (undiagnosed). • Presumed parasomnia/nocturnal seizure disorder does not respond to conventional treatment. • Presumed parasomnia is injurious to the patient or others (or is potentially injurious) or follows trauma or with forensic (legal) implications. • Presumed parasomnia has atypical features (stereotypic behavior, frequent events per night, atypical age of onset). MSLT = multiple sleep latency test; PSG = polysomnography; RLS = restless legs syndrome. TABLE 13–4 Summary of Circumstances in Which Polysomnography Is NOT Indicated PSG is the standard diagnostic study for evaluation of a suspected SRBD. A diagnostic study may be repeated if the initial study was negative for sleep apnea and there is a high clinical index of suspicion for this disorder. PSG should be performed preoperatively for planned surgery to treat snoring or suspected OSA.1,2 PSG is indicated for snoring because an appreciable number of patients will also have OSA. A PSG is indicated after surgery for OSA (after surgical healing) to document effectiveness. The practice parameters specify “in moderate to severe OSA,” although most clinicians would perform a PSG after surgery for mild OSA as well. Determination of the presence of OSA is indicated for planned OA treatment of snoring or suspected OSA.6 The type of diagnostic procedure was not specified in the practice parameters for OAs. However, PSG is always indicated to diagnose OSA. PSG is indicated after adjustment of an OA for OSA (not for primary snoring) to document efficacy. The 2007 practice parameters for PSG recommended a sleep study with the patient using an OA for treatment of moderate to severe OSA to document efficacy.2 In a subsequent practice parameter on OA treatment of OSA, a PSG to document efficacy was recommended for OSA of all severities.6 For patients with prior effective surgical treatment (documented by PSG), the PSG may be repeated at a later time if symptoms of sleep apnea return. For a patient using an OA as treatment for OSA, the PSG may also be repeated while the patient wears the OA if the patient’s symptoms return. If a patient on CPAP loses more than 10% of body weight, a diagnostic PSG is indicated to determine whether CPAP is still needed. This assumes the test is clinically indicated based on evaluation of the patient’s overall clinical status and the likelihood of weight loss maintenance. The 2005 practice parameters for PSG also mentioned a number of circumstances in which SRBDs are very common.2 However, PSG is NOT routinely indicated in those circumstances unless a clinical evaluation reveals a reasonable suspicion for SRBD. The disorders discussed included patients with systolic or diastolic heart failure, recent or past stroke or transient ischemic attack (TIA), coronary artery disease, and tachyarrhythmias or bradyarrhythmias. Most clinicians would also place resistant hypertension or pulmonary hypertension of unknown etiology in this category as well. The clinician should recognize that many patients with significant OSA do not complain of daytime sleepiness. A history of snoring and gasping would suggest a PSG is indicated. It should also be noted that some patients with OSA complain of insomnia. The practice parameters do list neuromuscular diseases as a group of disorders in which PSG is indicated for evaluation of sleep-related symptoms. Routine evaluation of chronic lung disease is not an indication for PSG unless coexistent OSA is suspected. Nocturnal oximetry is a useful tool for determining whether nocturnal oxygen desaturation is occurring in a patient with chronic obstructive pulmonary disease (COPD). A saw-tooth pattern is suggestive of sleep apnea. A PSG for PAP titration is the standard procedure to select a level of pressure for treatment.1,2,11 The titration can be performed on a separate night after a diagnostic PSG or during the second part of the night during a split (partial-night) study. A split sleep study is recommended when (1) the diagnostic portion shows an apnea-hypopnea index (AHI) greater than 40/hr with at least 2 hours of monitoring, (2) there is an AHI of 20 to 40 with special clinical circumstances such as severe desaturation or arrhythmia thought due to OSA, and (3) at least 3 hours remain for the PSG titration.1,2,11,12 If the PSG titration does not last at least 3 hours or is not adequate, a repeat PSG titration is indicated. PSG PAP titration techniques and PAP treatment are discussed in Chapter 19. Of note, financial constraints may require use of less stringent split study criteria. PSG is NOT recommended in patients on CPAP treatment who are doing well. If the patient is being treated on CPAP and is NOT doing well, a repeat PSG study on CPAP is indicated.2 However, before this expensive procedure, it is essential to document adequate objective adherence and to optimize treatment and the mask interface. PSG is also indicated if a patient on CPAP gains more than 10% of body weight to determine whether the pressure is adequate. However, a repeat PSG titration may not be clinically indicated. A PSG preceding an MSLT is indicated for evaluation of suspected narcolepsy or to help differentiate narcolepsy from idiopathic hypersomnia.1–3 PSG is indicated for evaluation of suspected periodic limb movement disorder but NOT the restless legs syndrome (RLS; a clinical diagnosis). A PSG with extended (and bilateral) electroencephalogram (EEG) derivations and video monitoring is indicated to evaluate (1) nocturnal behavior possibly due to seizures, (2) atypical parasomnia behavior (frequent episodes each night, stereotypic behavior, or behavior unusual for age), (3) nocturnal behavior/parasomnia that has resulted in injury to the patient or others (or has the potential to do so), (4) presumed parasomnia or nocturnal seizure disorder that does not respond to conventional treatment, or (5) if there are legal/forensic implications of nocturnal behavior.2 The practice parameters recommend looking at events in a 10-second window to observe for seizure activity and consultation with a physician with expertise in reading clinical EEGs if necessary. PSG is not indicated for evaluation of insomnia (unless OSA is suspected) or unless insomnia does not respond to usual treatment. PSG is not indicated for diagnosis of depression or insomnia with depression.2,4 The practice parameters for use of PSG4 in evaluation of insomnia state “PSG is indicated when initial diagnosis is uncertain, treatment fails (behavioral or pharmacologic), or precipitous arousals occur with violent or injurious behavior (Guideline).” Although the PSG finding of a short rapid eye movement (REM) latency is common in depression, this finding is not sufficiently sensitive or specific to warrant a PSG in evaluating patients with suspected depression. A PSG is NOT indicated for (1) typical uncomplicated and noninjurious parasomnias when the diagnosis is clearly delineated or (2) patients with known seizure disorders who have no nocturnal complaints. Table 13–4 is a summary of the circumstances in which PSG is not indicated. If a patient with OSA continues to have daytime sleepiness on adequate PAP treatment (documented adequate adherence and adequate control of respiratory events), there are two options. As discussed in Chapter 18 on medical treatment of OSA, an alerting agent such as modafinil may be added.13 If there is a suspicion of narcolepsy in addition to OSA, a nocturnal PSG on PAP treatment (documenting adequate treatment) followed by an MSLT on PAP can be performed (see Chapters 14 and 24). If unambiguous cataplexy is present, the patient likely has narcolepsy and the MSLT is simply confirmatory. It is essential that adequate PAP treatment for OSA be confirmed before expensive testing. This includes documentation of adequate objective adherence. Many PAP devices also have the ability to record residual AHI. A high residual AHI would be an indication for an adjustment in pressure (empirical increase) or a PSG PAP titration.1,2 The PAP device estimate of residual AHI is not always accurate, but a high value has a reasonable positive predictive value that the residual AHI is indeed elevated.14 A surprising number of patients (up to 15%) on chronic PAP treatment are not adequately treated (AHI > 10/hr).15,16 Before the PSG is read, a review of the clinical history with special attention to symptoms of sleep apnea, narcolepsy, RLS, and medications is very useful. The amount of chronic alcohol intake and the medications actually taken before the sleep study should be noted. The presence of underlying lung disease may help explain a low awake arterial oxygen saturation (SaO2) or low baseline sleeping SaO2. A clinical history of pacemaker insertion or known atrial fibrillation is also very helpful in providing a useful interpretation of ECG findings. If a PAP titration is planned for a patient currently using CPAP, the current treatment pressure level should be noted. During the reading of the PSG, a return to the clinical history may be helpful (Table 13–5). TABLE 13–5 Historical Elements to Review Based on Polysomnography Findings ? = consult history for relevant items. All digital PSG systems have a view that shows graphical summary information of the entire night (Fig. 13–1 and Table 13–6). It is often useful to look at the big picture before going through the data in smaller time windows. The biocalibrations are often helpful in noting the appearance of eyes-open wake in a given patient and whether the patient produces an alpha rhythm with eye closure (see Chapters 3 and 4). TABLE 13–6 Special Elements of Polysomnography to Note during Interpretation PM, also called home sleep testing (HST) or out of center sleep testing (OCST), has been the subject of intense interest recently.17,18 The tests are not always performed in the home; hence, the terms HST or PM are not ideal but are used in much of the literature on this subject. The term PM is used in this chapter. In the past, PM has been used to diagnose OSA in settings in which access to PSG is limited or delayed. The traditional classification of monitoring devices for the diagnosis of sleep apnea was originated by Ferber and colleagues (Table 13–7).19–21 This classification was used for the Tri-Society PM evidence review,22 executive summary,23 and practice parameters for the PM.7 The original classification used “level I, II, III, and IV” to refer to different classes of monitoring but currently the terminology is “type 1, 2, 3, and 4.” The Centers for Medicare and Medicaid Services (CMS) has a different classification for monitoring (Table 13–8).9 The CMS terminology defines the respiratory disturbance index (RDI) as the total number of apneas and hypopneas per hour of monitoring time. Therefore, the index determined by PM (no EEG) would be an RDI using the CMS definition. CMS also refers to PM as HST. TABLE 13–7 Classification of Portable Monitoring* *Levels I, II, III, and IV monitoring are now termed types 1, 2, 3, and 4. From Ferber R, Millman R, Coppola M, et al: ASDA standards of practice: portable recording in the assessment of obstructive sleep apnea. Sleep 1994;17:378–392. TABLE 13–8 Centers for Medicare and Medicaid Services Classification of Portable Monitoring Important developments in the history of PM are listed in Table 13–9. AASM practice parameters in 1994,20 1997,1 and 20052 specified a limited role for attended PM. However, unattended PM was widely used in locales in which access to PSG was very limited. On the basis of a Tri-Society evidence review for the use of PM,22 practice parameters published in 20037 stated that certain type 3 PM devices used in the attended setting could be used to rule in or rule out OSA.7 Before 2008, the National Carrier Determination (NCD) 240.4 on CPAP treatment specified a diagnosis of OSA must be made by attended PSG for subsequent CPAP treatment to be reimbursed. There were several requests for CMS to revise the policy to include PM (HST) devices. The evaluation of PM changed from an analysis of accuracy of PM compared with PSG to an analysis of PM’s ability to identify OSA patients who would benefit from treatment. In 2007, the AASM published clinical guidelines for the use of unattended PM8 (PMGs) (Appendix 13–2). In a 2008 ruling, CMS issued a decision allowing patients to qualify for CPAP treatment on the basis of a diagnosis by HST provided certain guidelines are followed.9 The specific rules vary according to local carrier determinations (LCDs; see Appendix 13–3, for example). In general, a physician Board-certified or Board-eligible (BC/BE) in sleep medicine or associated with a sleep center accredited by the AASM or the Joint Commission is allowed to interpret the HSTs. The durable medical equipment (DME) providers are not allowed to perform HST on patients that they will provide with PAP equipment. In 2009, CMS published a decision stating that PM testing itself would be reimbursable.10 The reimbursement fees vary between locales but are very modest. The AASM now offers accreditation for OCST. TABLE 13–9 History of Recent Developments in Portable Monitoring (Home Sleep Testing) Attended cardiorespiratory study (type 3) is an acceptable alternative to PSG if: • High pretest probability of OSA. • A negative type 3 study with patients with high pretest probability of OSA is followed by a PSG. Here, type 3 is defined as airflow, effort, oximetry, and ECG or heart rate. Attended cardiopulmonary study acceptable: • Preoperative for planned surgery for snoring or OSA. • After surgery for OSA—moderate to severe. • To document effectiveness of an OA. • After surgical or dental treatment of OSA and symptoms return. Attended cardiorespiratory study NOT recommended for PAP titration. The analysis of agreement between measurements from different devices is complex.22,24 A systematic review of agreement between PSG and ambulatory sleep studies was published in 2003.22 At that time, the best evidence for agreement between PM and PSG was for type 3 PM studies in the attended setting. The analysis focused on comparison of AHI values. However, the major issue is ability of the PM-derived AHI to correctly classify patients as having OSA and identifying those patients who would benefit from treatment. For example, AHI results of 30/hr versus 60/hr would both be consistent with severe sleep apnea even though the AHI values were quite different. Conversely, AHI results of 3 versus 13 would classify the patient as either being normal or having mild OSA. Numerous studies have compared the AHIs derived from PSG and PM devices. There are a number of reasons why the AHIs from PSG and PM might differ (Table 13–10). In general, studies comparing PSG and PM have either used simultaneous monitoring with PSG and PM in the sleep center or compared PSG in the sleep center with unattended PM on a different night at home. Both approaches are problematic. In either case, even if the same type of sensors are used and the same number of events are identified, the two devices would still give different AHI values as monitoring time (PM) would exceed total sleep time (PSG). For example, if both methods detect 70 apneas and hypopneas, dividing by 5 hours of total sleep time (TST; AHI = 14/hr) will give a higher index than dividing by 7 hours of monitoring time (AHI = 10/hr). It is also possible that the oximeters used in different systems could differ in their ability to detect desaturations. Comparing PM and PSG AHI values during simultaneous recording reduces the effects of night-to-night variability. However, this does not mimic how the devices are actually used. The different nights–different locations approach mimics real world conditions. However, if the different nights approach is used to compare PSG and PM, the AHI values could differ simply due to night-to-night variability in the AHI. TABLE 13–10 Reasons for Difference in Apnea-Hypopnea Index by Polysomnography versus Portable Monitoring • Monitoring time > TST (i.e., 10/5 < 10/2). • PM sensors dislodged during the night. • Different scoring (hypopnea with arousals only with PSG).
Polysomnography, Portable Monitoring, and Actigraphy
Polysomnography
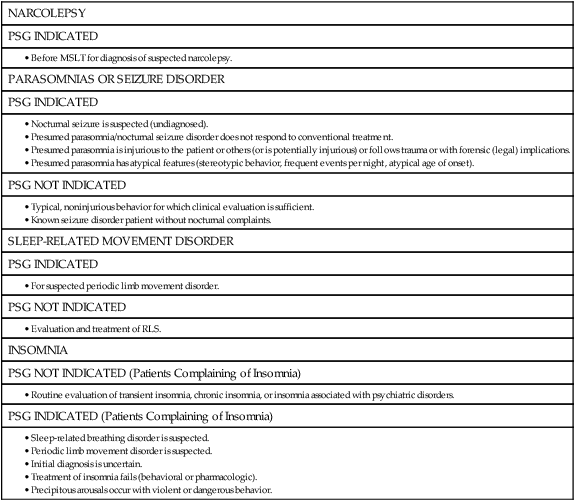
DIAGNOSTIC PSG
REPEAT PSG
FOLLOW-UP PSG (NON-PAP TREATMENT)
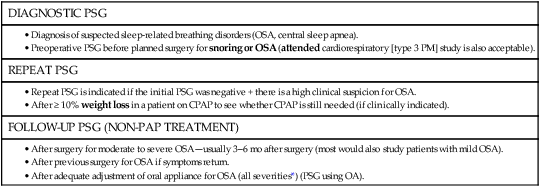
PAP TITRATION
REPEAT PSG ON CPAP
REPEAT PSG ON CPAP NOT INDICATED
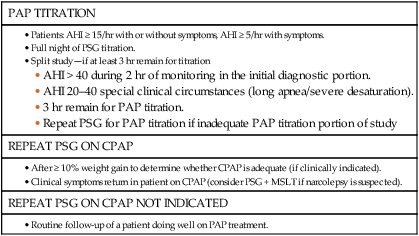
NARCOLEPSY
PSG INDICATED
PARASOMNIAS OR SEIZURE DISORDER
PSG INDICATED
PSG NOT INDICATED
SLEEP-RELATED MOVEMENT DISORDER
PSG INDICATED
PSG NOT INDICATED
INSOMNIA
PSG NOT INDICATED (Patients Complaining of Insomnia)
PSG INDICATED (Patients Complaining of Insomnia)

PSG for Patients with SRBDs (see Table 13–1)
PSG Titration and PSG on CPAP (see Table 13–2)
PSG Indications: Nonrespiratory Disorders
PSG Indicated (see Tables 13–3 and 13–4)
PSG NOT Indicated (see Tables 13–3 and 13–4)
Approach to the Sleepy Patient on PAP Treatment
Approach to Reading the PSG
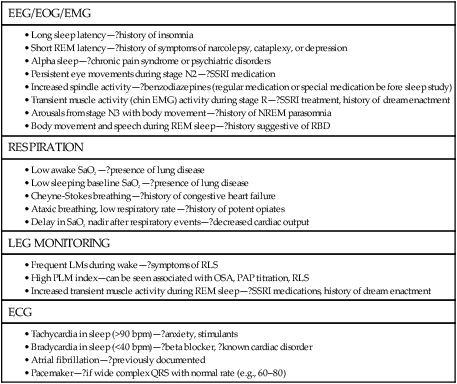

Portable Monitoring (home sleep testing, out of center sleep testing)
TYPE 1: ATTENDED PSG
TYPE 2: UNATTENDED PSG
TYPE 3: MODIFIED PORTABLE SLEEP APNEA TESTING
TYPE 4: CONTINUOUS SINGLE OR DUAL BIOPARAMETER RECORDING
Measures (channels)
Minimum of seven channels including ECG, EEG, EOG, chin EMG, airflow, respiratory effort, oxygen saturation
Minimum of seven channels including EEG, EOG, chin EMG, heart rate or ECG, airflow, respiratory effort, oxygen saturation
Minimum of four, including ventilation (at least two channels of respiratory movement or respiratory movement and airflow), heart rate or ECG, and oxygen saturation
Minimum of one oxygen saturation, flow, or chest movement
Body position
Documented or objectively measured
Possible
Possible
No
Leg movement
EMG or motion sensor desirable but optional
Optional
Optional
No
Personnel interventions
Possible
No
No
No
HCPCS CODE
TYPE
SETTING
MONITORING
G0398
2
Unattended
Minimum of seven channels including EEG, EOG, EMG, ECG/heart rate, oxygen saturation, anterior tibial EMG
G0399
3
Unattended
Minimum of four channels and must record ventilation, oximetry, and ECG or heart rate
G0400
4
Unattended
Minimum of three channels, and one must be airflow
—
Unattended
Minimum of three channels including peripheral arterial tonometry, actigraphy, and oximetry

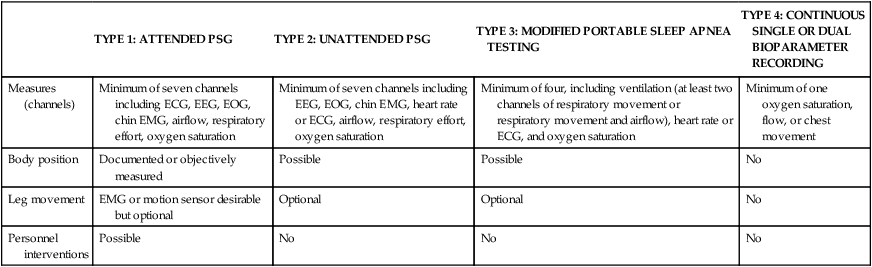
Developments in the Use of PM
1994 Practice Parameter for Portable Monitoring20
Tri-Society (ACCP, ATS, AASM) 2003
Evidence Review (Chest)22
Executive Summary23
(Am J Respir Crit Care Med)
PM Practice Parameters (Sleep)24
Attended type 3 studies may be used to rule in or rule out OSA provided certain limitations are met. These include manually scoring the records, using the device in patients without significant co-morbid conditions, not using type 3 devices for PAP titrations or split-night studies, and symptomatic patients who have a negative type 3 study should have a PSG.
Type 2, type 4 studies not recommended.
2004 CMS Decision
Insufficient evidence to recommend unattended type 2, 3, 4 PM.
2005 AASM Practice Parameters for PSG and Related Procedures2
AASM Portable Monitoring Task Force 2006–2007
Clinical Guidelines for Unattended Monitoring 20076
CMS 240.4 Final decision 2008–20099,10
Local Carrier Determinations
Accuracy of PMs
PM AHI > PSG AHI
PSG AHI > PM AHI
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access