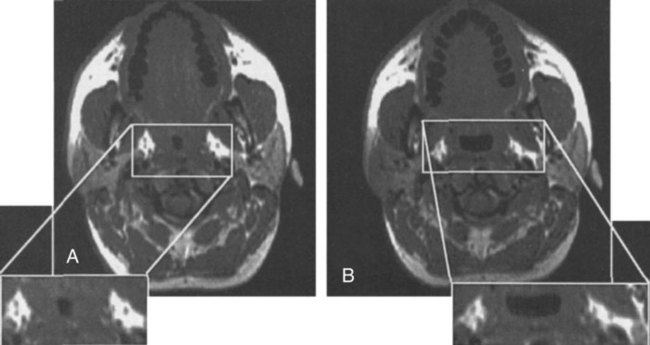
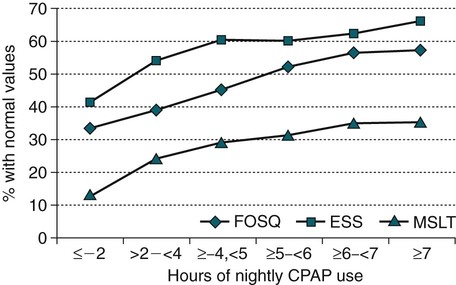
• PAP is the treatment of choice for patients with moderate to severe OSA and is an option in symptomatic patients with mild OSA. • Although many variants of PAP have been developed, CPAP remains the treatment of choice for most OSA patients. • BPAP devices deliver separately adjustable IPAP and EPAP with IPAP > EPAP. • APAP devices provide the lowest effective pressure for a given posture and sleep stage. • BPAP, APAP, or flexible PAP devices have not significantly improved adherence to PAP treatment for unselected patients. However, individual patients may benefit from these treatment modes. • BPAP in the spontaneous mode is used for pressure intolerant OSA patients and to deliver pressure support (PS = IPAP − EPAP). • BPAP in the ST mode (backup rate) is used for NPPV treatment of patients who have central apneas or central ventilatory control disorders. • BPAP-ST is also used for patients who may not reliably trigger a transition for EPAP to IPAP due to neuromuscular or chest wall disorders. • ASV is used for patients with an instability in ventilatory control manifested by CSB-central apneas, central apneas from narcotics, or CompSA of unknown etiology that does not improve with chronic CPAP treatment. • PAP treatment is very effective at reversing or improving many manifestations of OSA. However, acceptance and adherence are major problems. • There is a dose response to CPAP usage with the Epworth Sleepiness Scale improving with as little as 4 hours of use but objective improvement in sleepiness and quality of life measures usually require longer CPAP use for maximal benefit. • Objective monitoring of adherence is essential for successful PAP treatment. This should be performed early after treatment initiation and at continuous intervals. • Documentation of adequate objective adherence is the first step to evaluate persistent sleepiness despite PAP treatment. • A PSG PAP titration is the standard approach for choosing an effective pressure. • Higher CPAP is needed in the supine position and during REM sleep. • Unattended APAP titration can be used to determine an effective level of CPAP (90th or 95th percentile pressure). • Chronic treatment with APAP devices in properly selected patients eliminates the need for a PSG titration but does not significantly improve adherence. Since the original description of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea (OSA) by Sullivan, Issa, and Berthon-Jones in 1981,1 positive airway pressure (PAP) remains the mainstay of treatment for moderate to severe OSA in adults.2–5 Despite many advances in technology, the major challenge facing clinicians is improving adherence to PAP treatment.6 PAP works by splinting the upper airway open during sleep.7–9 Studies have shown CPAP to increase upper airway size, especially in the lateral dimension (Fig. 19–1). Positive intraluminal pressure expands the upper airway (pneumatic splint). This is a passive process because upper airway muscle activity is reduced by CPAP.10 Although the pneumatic splint is the main mechanism of action, an increase in lung volume due to CPAP may also increase upper airway size and/or stiffen the upper airway walls, making them less collapsible. In general, upper airway size increases as lung volume increases.11 This is thought due to a downward pull on upper airway structures during lung expansion (“tracheal tug”).12 Whereas CPAP maintains a positive intraluminal pressure during both inspiration and expiration, one study using only expiratory positive airway pressure (EPAP) showed a reduction in respiratory events.13Another study was not able to reproduce a beneficial effect of EPAP.14 This could be due to differences in study design or the method of delivery of EPAP (threshold valve vs. expiratory resistance). Numerous studies have shown that PAP can bring the apnea-hypopnea index (AHI) down to below 5 to 10/hr in the majority of patients.2 The virtual elimination of apnea and hypopnea improves arterial oxygen saturation and decreases respiratory arousals. In some patients, PAP treatment can also increase the amount of stage N3 and stage R (rapid eye movement [REM] sleep). An occasional patient with very severe apnea will have a large REM or stage N3 sleep rebound on the first night of PAP treatment. This is most commonly seen when an entire night of polysomnography (PSG) is available for PAP titration. The most difficult problem with PAP treatment is that adherence is suboptimal in a large percentage of patients.6 Many of the benefits of PAP treatment are discussed in Chapter 17. A number of modes of delivering PAP exist (Table 19–1). CPAP delivers a predetermined constant pressure during both inspiration and exhalation (Fig. 19–2). Bilevel positive airway pressure (BPAP) delivers separately adjustable higher inspiratory positive airway pressure (IPAP) and lower EPAP (see Fig. 19–2).15 Of note, in unselected patients, BPAP treatment does not result in higher rates of adherence than CPAP.16 A Cochrane database analysis of six studies and 285 participants found no significant difference in usage with BPAP compared with CPAP.17 However, some patients failing CPAP will tolerate BPAP.18,19 This is especially true of patients having difficulty exhaling or with complaints of bloating. The IPAP-EPAP differential (pressure support [PS]) is useful for augmenting ventilation in patients with OSA and concomitant hypoventilation. These groups include the obesity hypoventilation syndrome (OHS) and the “overlap syndrome” (OSA + chronic obstructive pulmonary disease [COPD]). Some patients with the OHS or the overlap syndrome can be adequately treated with CPAP alone.20,21 However, other patients in this group require BPAP, especially if significant hypoventilation is present. TABLE 19–1 Modes of Positive Airway Pressure Devices BPAP is also used for noninvasive positive pressure ventilation (NPPV) in chronic alveolar hypoventilation syndromes.22 In patients with OSA and OHS, BPAP is usually used in the spontaneous (S) mode. In this mode, the patient cycles the device from EPAP to IPAP and sets the respiratory rate. BPAP is also available with a backup rate (spontaneous-time [ST] mode) or a set respiratory rate (timed [T] mode). These modes are used to provide NPPV to patients who unreliably cycle the device between IPAP and EPAP due to muscle weakness or abnormal central ventilatory control (central apnea). In the ST mode, the device will provide a machine-triggered breath with the specified IPAPtime (inspiratory time) if no spontaneous breath occurs within a time window. For example, if the backup rate is 12 breaths/min, there is a 5-second window following the start of the last breath. If no spontaneous breath occurs during the time window, the machine will deliver a machine-triggered breath (Fig. 19–3). In the T mode, the device delivers IPAP/EPAP cycles with a set IPAPtime at a specified respiratory rate. For example, with a rate of 20, the cycle time is 3 seconds. If IPAPtime = 1 sec, then EPAP time = 2 sec. The IPAP time is usually set at 1.2 to 1.6 seconds depending on the backup rate. Autoadjusting (autotitrating) positive airway pressure (autoCPAP, autoPAP, APAP) devices were developed with two potential uses: (1) autotitrating PAP to select an effective level of CPAP without the need for an attended titration and (2) autoadjusting PAP for chronic treatment with the advantage of delivering the lowest effective pressure in any circumstance.23–26 Chronic treatment with APAP would also eliminate the requirement for a CPAP titration. When APAP was first developed, improvement in PAP adherence was a goal. Although there are conflicting data, one meta-analysis showed that APAP does not improve adherence compared with CPAP.27 A more recent larger meta-analysis of 30 studies and 1136 participants found a statistically significant difference in machine usage of 0.21 hour (12 min), which is not clinically significant.17 However, individual patients may tolerate APAP better than CPAP. The APAP algorithms vary between different devices, but in most instances, the pressure changes in response to variations in airflow magnitude (apnea or hypopnea), airflow limitation (flattening of the airflow contour), snoring (vibration), and/or airway impedance.23 The pressure changes gradually between the preset lower and upper pressure limits (Pmax, Pmin) to avoid inducing arousal. If none of the monitored variables is detected, the device slowly lowers the pressure to a minimum effective setting. The pressure varies during the night in response to changes in body position or sleep stage that may alter the pressure required to maintain an open upper airway (Fig. 19–4). The average pressure is typically only 2 to 3 cm H2O lower than the fixed pressure that would be effective during the entire night but can be up to 6 cm H2O lower.28 Of note, different brands of devices may respond very differently to changes in airflow.29 High air leak (mask or mouth leak), which simulates physiologic events, and inability to differentiate between central and obstructive apnea by these devices can result in errors in APAP titration.23,30 The APAP devices have no method of determining whether inspiratory effort is present during an apnea. In the past, some autotitration algorithms would not titrate above 10 cm H2O unless snoring or airflow limitation was present. Other algorithms would not continue to increase pressure if this did not reduce apnea (nonresponsive apnea). New technology used by Philips-Respironics attempts to differentiate “clear airway apneas” versus obstructive apneas by delivering a small pressure pulse (1–2 cm H2O pressure pulse) after approximately 6 seconds of a reduction in airflow (Fig. 19–5). If the pressure pulse does produce an increase in flow, this is compatible with an open airway (clear airway). If the pressure pulse does not increase flow, the airway is closed. An APAP device using this technology does not increase pressure for “clear airway” apneas. Note that a closed airway can occur with some central apneas.31 Thus, using this approach, some apneas classified as obstructive could actually be closed airway central apneas. The use of APAP devices for autotitration is discussed in more detail in later sections. An autoBPAP device is also available (Fig. 19–6). The physician sets the minimum EPAP, and the minimum and maximum pressure support (IPAP-EPAP), as well as the maximum IPAP. The minimum PS is 3 cm H2O by default. The machine then adjusts both the EPAP and the IPAP to maintain an open airway. The advantages of autoBPAP over other PAP modes remain to be demonstrated. A recent study found autoBPAP to be useful as “rescue” therapy in patients not compliant to CPAP treatment in spite of the usual interventions.32 Thus, autoBPAP could potentially be useful in very pressure-intolerant patients who find BPAP alone unacceptable or in patients for whom an effective bilevel pressure is not known. From Kakkar RK, Berry RB: Positive airway pressure treatment for obstructive sleep apnea. Chest 2007;132:1057–1072. Adaptive servoventilation (ASV)33–38 is a variant of BPAP that was developed to treat Cheyne-Stokes central apnea in patients with congestive heart failure.33,37,38 Both ASV and BPAP devices with a backup rate are approved for use with patients with central apnea and complex sleep apnea (CompSA; central apnea that persists or appears during a PAP titration). ASV devices attempt to stabilize ventilation in patients with ventilatory instability such as Cheyne-Stokes breathing (CSB),33,37,38 narcotic-induced central apnea,36 and CompSA of unknown etiology.34,35 During an ASV titration, a level of EPAP is chosen to keep the upper airway open (preventing obstructive apnea) and the IPAP-EPAP difference (PS) automatically adapts between minimum and maximum levels to stabilize ventilation (Fig. 19–7). ASV devices from two manufacturers are available in the United States (Table 19–2). They both adjust pressures with a goal of stabilizing ventilation. The ResMed device (VPAP Adapt SV) uses a goal of providing 90% of the recent average ventilation (moving time window). With low tidal volume, the PS increases, and with high tidal volume, the PS decreases. An automatic backup rate triggers an IPAP/EPAP cycle if central apnea is present (default 15 breaths/min). The technologist can only adjust the EPAP (termed “EPP” for this brand of ASV). The PS is constrained by PSmax (≥10 cm H2O is recommended) and the maximum IPAP is constrained by maximum pressure of 25 cm H2O. TABLE 19–2 Adaptive Servoventilation Devices Of note, patients with CSB-central apnea and CompSA (including patients on narcotics) can also be treated with BPAP in the ST mode. One study comparing BPAP-ST with ASV in patients with central apnea and CompSA found ASV to result in only a slightly lower AHI than BPAP-ST.34 However, stabilizing ventilation with BPAP-ST in this situation often requires a high backup rate (with a high proportion of machine-triggered breaths). Higher PS is usually needed to deliver a machine-triggered breath because the patient does not assist with inspiration. It is preferable to have the patient trigger the majority of breaths because timing and effective ventilation are usually better. Thus, ASV is generally preferable because, if ventilation is stabilized, a majority of the breaths will be patient-triggered. However, there are individual patients who may respond better to BPAP-ST. An important concept when using ASV is that of closed airway central apnea. As noted previously, during central apnea, the upper airway may close.31 For example, during a mixed apnea, the airway has closed during the central portion. If a closed airway central apnea occurs, the machine-triggered PS will not effectively deliver flow (or tidal volume). In this case, higher EPAP is needed (Fig. 19–8). ASV was not specifically designed for patients with nocturnal hypoventilation. However, some patients with CompSA due to narcotics have both hypoventilation and instability in breathing. A higher PSmin (≥4 cm H2O) may be needed if the arterial oxygen saturation (SaO2) remains low but breathing is regular. Another situation in which PSmin greater than 0 is useful is the pressure-intolerant patient. Rather than using EPAP of 16, one could use an EPAP of 14 and a PSmin of 4. Thus, the device would deliver a basal pressure of 18/14 cm H2O with higher IPAP as needed to stabilize ventilation. This example also illustrates that if a patient requires high EPAP, quite high IPAP values may be needed to deliver an adequate PS. Recently, volume-targeted bilevel positive airway pressure (VT-BPAP) has been developed in which the IPAP-EPAP difference is automatically adjusted to deliver a target tidal volume.39–42 VT-BPAP has the potential advantage of automatically varying the PS to deliver a targeted tidal volume if the condition of the patient changes. For example, if respiratory muscle strength declined and the tidal volume decreased, the device would deliver higher PS to return the delivered tidal volume to the targeted level. VT-BPAP can be used in the S, ST, or T mode. Relatively few studies on VT-BPAP devices have been published. To date, only one VT-BPAP device is available in the United States (Average Volume Assured Pressure Support [AVAPS], Philips-Respironics). Storre and colleagues39 compared BPAP and AVAPS (both in the ST mode) using a randomized, cross-over trial in patients with OHS. AVAPS resulted in a slightly higher ventilation and lower arterial partial pressure of carbon dioxide (PaCO2) without any better sleep quality or quality of life measures compared with BPAP-ST. Using the same device, Ambrogio and associates40 studied an assortment of patients with chronic alveolar hypoventilation who were stable on BPAP support. A validation night preceded the study nights to ensure the BPAP settings were adequate and to provide guidance for the AVAPS settings. Patients then were studied on BPAP or AVAPS in random order. On AVAPS, the minute ventilation was greater than on BPAP but sleep quality was comparable between the two NPPV modes. Using a different VT-BPAP device, Janssens and coworkers41 monitored patients with the OHS using either BPAP or VT-BPAP for one night each in random order. Sleep quality was worse but nocturnal transcutaneous partial pressure of carbon dioxide (TcPCO2) was slightly lower on VT-BPAP. It is possible that patients might have exhibited better sleep quality after a longer period of adaptation to the VT-BPAP. Jaye and colleagues42 compared BPAP-ST with an autotitrating bilevel ventilator (autoVPAP) in patients with stable neuromuscular and chest wall disease with nocturnal hypoventilation and found autoVPAP to produce comparable control of nocturnal oxygenation without compromising sleep quality. The mean nocturnal TcPCO2 was higher with autoVPAP, although the difference was unlikely to be clinically significant. AutoVPAP (ResMed, Poway, CA) is not currently available in the United States. These studies suggest VT-BPAP can be effective, but the role of VT-BPAP in the NPPV treatment of patients with chronic alveolar hypoventilation syndromes remains to be determined. Two manufacturers of PAP devices have developed flexible PAP in an attempt to improve patient comfort and adherence. Some PAP devices manufactured by Philips-Respironics provide several comfort options (Cflex, Cflex+, and Aflex) (Fig. 19–9). ResMed devices offer expiratory pressure relief (EPR). However, there are no convincing data that any of these options improve adherence in unselected patients. In Cflex, expiratory pressure drops at the start of exhalation but returns to the set CPAP at end-exhalation. The amount of drop (Cflex 1, 2, 3) is determined by a proprietary algorithm. In general, there is a greater pressure drop for greater flow during exhalation. Cflex is available on APAP devices as well as CPAP. Cflex+ adds a smoothing of the transition from inhalation to exhalation. Aflex is a form of APAP that provides a 2 cm H2O lower end-expiratory pressure than the inspiratory pressure (in addition to the features of Cflex+). For both BPAP and autoBPAP devices, a form of expiratory pressure relief is available (Biflex). The technology provides a smoothing of transition from IPAP to EPAP as well as expiratory pressure relief during the EPAP cycle (Biflex 1, 2, 3). An initial study found that flexible PAP improved adherence by about Today, most PAP devices come with the option of an integrated heated humidification system. They can be used in the cool humidity mode if desired. Heated humidity (HH) can deliver a greater level of moisture than cool humidification and may be especially useful in patients with mouth leak or nasal congestion. Mouth leak can cause a dramatic fall in relative humidity50 (Fig. 19–10) and a loss of humidity from the upper airway/CPAP system, thus drying the nasal or oral mucosa. Drying of the nasal mucosa increases nasal resistance and this is minimized by use of HH.51,52 HH is more effective than cool at delivering moisture. The level of humidity can be adjusted by the patient to meet variable needs. An occasional patient will prefer cool humidity (heat turned off) or no humidity at all. Adequate cleaning of the humidifier chamber and hoses does require extra patient effort. A number of technologic advances for adjusting humidity to prevent rain-out in the tubing and mask have recently been introduced. One study suggested that use of humidity is associated with an increase in risk of infectious complications53 that can be reduced with use of a filter.54 There is an occasional patient with recurrent sinus infections who seems to do better without humidification—but the etiology of this improvement is unclear. Studies determining whether HH improves either acceptance of CPAP after titration or long-term adherence to CPAP treatment have found conflicting results. Massie and associates55 studied patients who received either HH or cool humidity for 3 weeks (random order), a 2-week washout period of no humidity, and 3 weeks of the alternative humidity. Patients on HH had about TABLE 19–3 Effect of Humidity on Outcomes Data from Massie CA, Hart RW: Clinical outcomes related to interface type in patients with obstructive sleep apnea/hypopnea syndrome who are using continuous positive airway pressure. Chest 2003;123:1112–1118. †P < .05. Heated humidity versus no humidity. When CPAP devices became available commercially, the first interfaces were nasal masks. Today, a large variety of interfaces is available (Fig. 19–11), but it may still be difficult to obtain a good mask fit. Nasal pillow masks are often better tolerated than traditional nasal masks by patients with claustrophobia and are useful in patients with a mustache or edentulous patients who have no dental support for the upper lip.63 It is essential to use a size of pillow large enough to provide a good seal. A wide variety of nasal masks with gel or air cushion interfaces is available. For patients who have severe nasal congestion or open their mouths during PAP treatment, oronasal (full face masks)64,65 and oral interfaces66,67 are available. Oronasal masks have to seal over a large area, and this makes finding a good fit very difficult in some patients. In edentulous patients, oronasal masks may also compress the soft tissues. Patients tend to overtighten masks, and this can cause damage to the nasal bridge or actually impair the ability of the mask to seal. Often, a trial of several masks is needed to find one that patients can use comfortably. This is a challenge because insurance providers typically will pay for only one mask every 3 to 6 months. This is one situation in which trying several different types of mask in the sleep center before the titration can be very useful. Adequate care and replacement of masks are also essential to maximize their ability to seal. If the patient gets up to use the bathroom during the night, we encourage disconnection of the hose from mask rather than taking off the mask. Masks that are removed in the middle of the night are often not replaced. Important elements of the AASM practice parameters for the use of PAP and APAP are listed in Appendix 19–1. Treatment with PAP is indicated for patients with moderate to severe OSA (with or without symptoms).2,3 PAP treatment is an option for patients with mild OSA who are symptomatic and choose PAP over other treatment options (upper airway surgery or an oral appliance). The Centers for Medicare and Medicaid Services (CMS) requirements for PAP reimbursement are specified in the National Carrier Determination 240.4.68 Patients must be diagnosed with either a PSG or a home sleep test (HST) that follows Medicare requirements for those evaluations (discussed in Chapter 13). CPAP treatment is reimbursed for an initial 12-week period if the AHI is 15/hr or greater with or without symptoms or if the AHI is 5/hr or greater but less than 14/hr if certain symptoms (excessive daytime sleepiness, impaired cognition, mood disorders, or insomnia) or certain disorders (hypertension, ischemic heart disease, or history of stroke) are present. For continued payment after that period, adequate objective adherence must be documented. For at least 1 month during the qualifying period, CPAP must be ≥4 hours for ≥70% of nights. A face-to-face evaluation of the patient by the physician ordering CPAP and documentation of a benefit from treatment are also required. The exact requirements vary with different local carrier determinations (LCDs) (Appendix 19–2). Most other health insurance providers follow similar guidelines. As noted in Chapters 18 and 20, the initial treatment for most children with OSA is tonsillectomy and adenoidectomy. However, residual sleep apnea is present in a significant portion of patients.69 The clinician must then decide on further treatment recommendations based on the residual AHI and symptoms. Excessive daytime sleepiness, poor school performance, behavioral issues, and cardiovascular consequences should all be considered. PAP is an effective option provided that both the parents and the patient accept this treatment. The exact AHI cutoff is not well defined, but many pediatricians consider a residual greater than 5/hr as indicating significant OSA in children and an indication for CPAP treatment. Those less symptomatic patients with an AHI between 1 and 5 are in a gray zone and treatment must be individualized. Starting CPAP in children often requires periods of adaptation to a mask before a titration can be attempted.62,70 Other options include weight loss, dental procedures, or intranasal steroids and leukotriene modifier therapy.71 Adherence rates are defined in many ways. Most devices compute the percentage of days used, the average use all days (averaging in 0s for days not used), average nightly use (days used), and the percentage of nights used ≥4 hours. An early paper reporting measurement of objective adherence by Kribbs and coworkers72 defined regular users as those who used CPAP at least 4 hr/day on at least 70% of nights. In their study, only 46% of patients met this criterion. There has been a tremendous variability in the reported rates of PAP adherence. This is due to a number of factors including different populations (moderate to severe OSA vs. all patients), different definitions of adherence, different length of follow-up, and different algorithms of initiating PAP treatment and following patients. One of the largest studies of long-term adherence with nasal CPAP reported only 68% of patients were still using CPAP at 5 years.73 Pépin and colleagues74 found 79% of patients using CPAP for longer than 4 hours on 70% of nights at 3 months. Sin and coworkers75 followed patients with an AHI greater than 20/hr and found greater than 85% were using the device longer than 3.5 hr/night at 6 months. Kohler and associates76 found that 81% of patients were using CPAP at 5 years. Several studies have addressed the factors associated with good versus poor PAP adherence (Table 19–4). In general, the factors identified to date explain relatively little of the large variance in CPAP acceptance and adherence.73,74,76,77 The level of pressure does not seem to be important. Finding an acceptable interface is often the biggest challenge in getting patients to adhere to PAP treatment. However, there is no evidence for the superiority of any type of interface. Although a great deal of effort is spent in intervening for side effects, the presence or absence of side effects does not seem to be a major determinant of PAP use.77 Patients may be willing to tolerate side effects if there is a significant improvement in pretreatment symptoms. Factors favoring better adherence include symptomatic daytime sleepiness, good response in sleepiness to treatment, and to a lesser extent disease severity (AHI). In one study a high arterial oxygen desaturation index predicted good adherence.76 Poor prognostic factors include spouse referral and high nasal resistance.75,78 Whether CPAP treatment follows a split night (diagnostic/PAP titration) or separate diagnostic and PAP titration studies does not seem to affect PAP adherence. Early adherence is a good predictor of long term PAP use. In a study of 32 patients followed for 9 weeks, the nightly duration of use differed between compliant and noncompliant patients by the fourth night of use.79 Budhiraja and colleagues80 found that long-term adherence to CPAP can be predicted as early as 3 days after CPAP initiation. Weaver and associates81 studied patients before and after 3 months of therapy and correlated objective adherence with improvement in functioning (Fig. 19–12). Thresholds above which further improvements were less likely relative to nightly duration of CPAP use were identified for Epworth Sleepiness Scale score (4 hr), multiple sleep latency test (6 hr), and Functional Outcomes Associated with Sleepiness Questionnaire (7.5 hr). Thus, as usage increases, subjective sleepiness, then objective sleepiness, and last, quality of life measures improve. The amount of necessary usage depends on what outcome is being evaluated. As noted above, the current Medicare guidelines state that devices will be reimbursed after 12 weeks only if objective adherence for a period of 1 month shows ≥4 hours of use for ≥70% of nights and the treating physician documents in a face-to-face meeting that the patient is benefiting from PAP treatment (see Appendix 19–2). However, many patients require much greater than 4 hours nightly use to experience a resolution of excessive sleepiness. Campos-Rodriguez and coworkers82 followed a historical cohort of 871 patients with OSA for a mean of 48 months. Five-year cumulative survival was highest in the group of patients who used PAP therapy more than 6 hours per night on average (96.4% survival) compared with patients who used PAP from 1 to 6 hours per night (91.3% survival) and patients who used PAP less than 1 hour per night (85.5%). The same group conducted a prospective cohort study of 55 patients with hypertension.83 Patients with average use greater than 5.3 hr/day and hypertension at entry to the study had a drop in mean arterial blood pressure of about 4 mm Hg. From Weaver TE, Maislin G, Dinges DF, et al: Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007;30:711–719. The literature on this subject is somewhat difficult to interpret because most programs use a number of interventions to try to improve adherence6 (Table 19–5). Although education about OSA and PAP treatment is recommended, there is no evidence that this dramatically improves adherence.84 Comprehensive programs of education (patient and bed partner), early contact, and interventions,85 a simple CPAP help line,86 or group education87 have improved adherence in some studies. Cognitive behavioral interventions have shown promise.88,89 Timely interventions for side effects and discomforts would also seem reasonable, although not proved to improve adherence. Table 19–6 presents common problems and possible solutions. Finding a comfortable and well-fitting mask interface is one of the biggest challenges in PAP treatment. Proper sizing and mask adjustment are essential and should be checked at every visit to the physician or respiratory therapist providing PAP support. If necessary, a different mask type could be tried. For mouth leaks, the addition of a chin strap, higher humidity, or a full face mask is an option. Sometimes, a leak will respond to slight lowering of pressure or switch from CPAP to BPAP if all else fails. Nasal pillows may help deal with claustrophobia. Unintentional mask removal may indicate a leak or inadequate pressure. Nasal congestion can be addressed with nasal steroids, antihistamines, increased humidity, or reduced mask leak. Rhinitis/rhinorrhea may respond to nasal ipratropium bromide. For pressure intolerance, use of a lower pressure, addition of flexible CPAP, a change to APAP or BPAP, and education about using the ramp are all options. TABLE 19–5 Methods to Improve Positive Airway Pressure Adherence OSA = obstructive sleep apnea; PAP = positive airway pressure. TABLE 19–6 Interventions for Common Positive Airway Pressure Treatment Side Effects Patients with both insomnia and OSA pose a difficult problem. In addition, some patients who normally have no problems with insomnia will have problems falling asleep or staying asleep on CPAP. Some clinicians have been hesitant to prescribe hypnotics, believing that this may reduce the effectiveness of CPAP. There has also been a concern that the use of alcohol could increase the required level of CPAP above that demonstrated to be effective in the sleep center. However, one study found that moderate alcohol consumption near bedtime did not impair the efficacy of CPAP.90 Another study found that zolpidem, a commonly used hypnotic, did not impair efficacy of a given level of CPAP.91 Conversely, it has been hypothesized that using a hypnotic might improve adherence to PAP in some patients. A study by Bradshaw and colleagues92 using zolpidem did not find an improvement. In contrast, Lettieri and associates93,94 found improvement during CPAP titration (sleep quality) and long-term adherence with eszopiclone (a hypnotic with a longer duration of action that zolpidem). It is possible that a longer-acting medication is needed to improve CPAP adherence. Although routine use of a hypnotic cannot currently be recommended, at least temporary use of a hypnotic should be considered if insomnia is a major obstacle to CPAP use.
Positive Airway Pressure Treatment
Mechanism of Action
Effectiveness
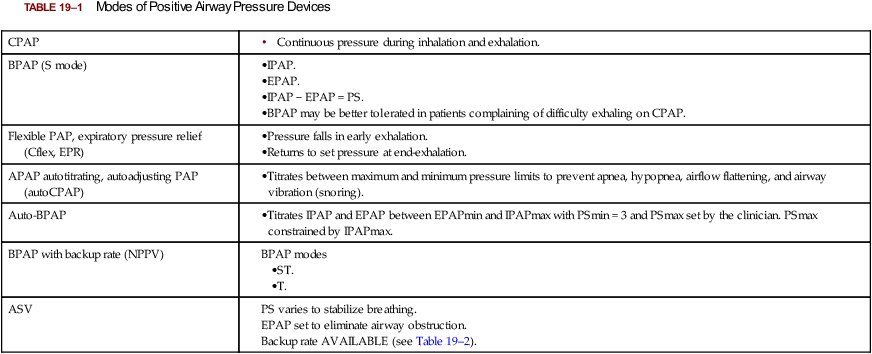
Modes of PAP
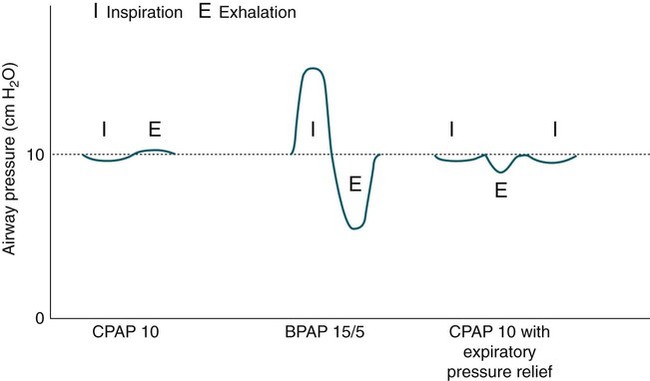
CPAP
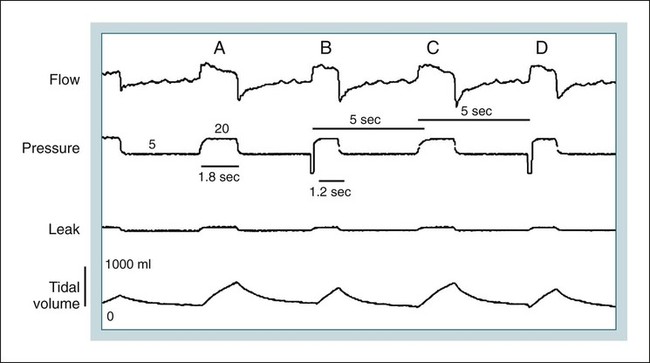
BPAP (S mode)
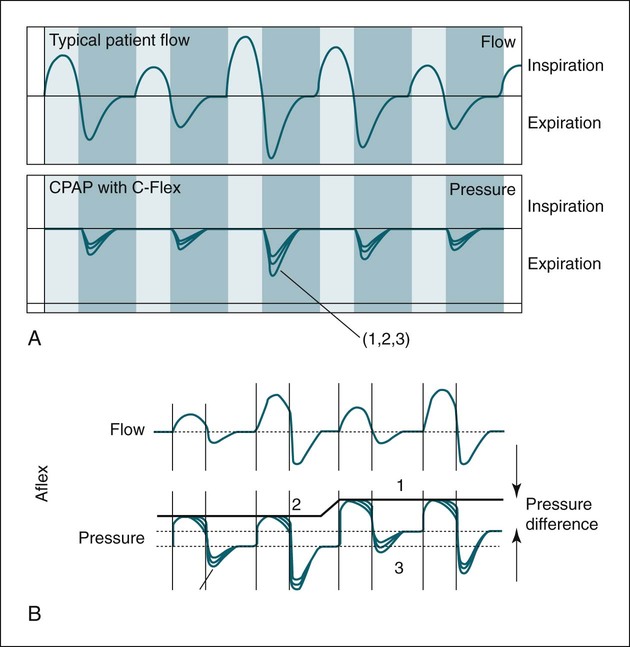
Flexible PAP, expiratory pressure relief (Cflex, EPR)
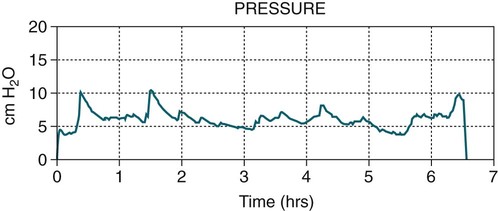
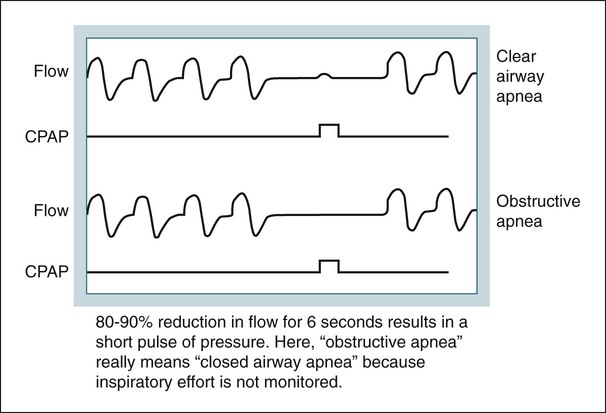
APAP autotitrating, autoadjusting PAP (autoCPAP)
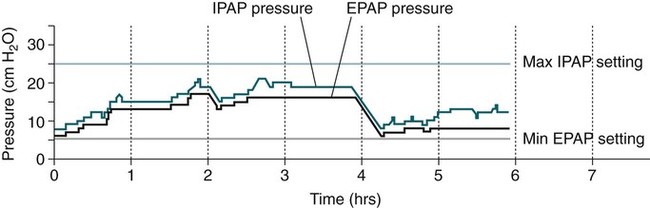
Auto-BPAP
BPAP with backup rate (NPPV)
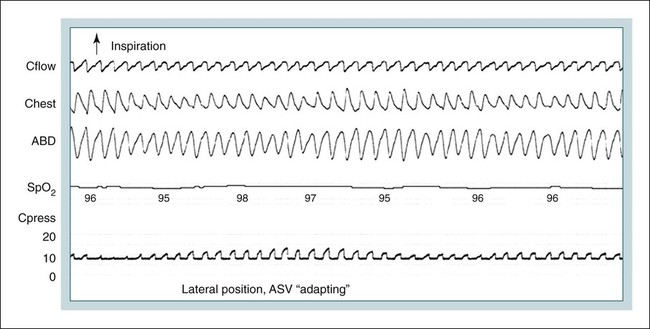
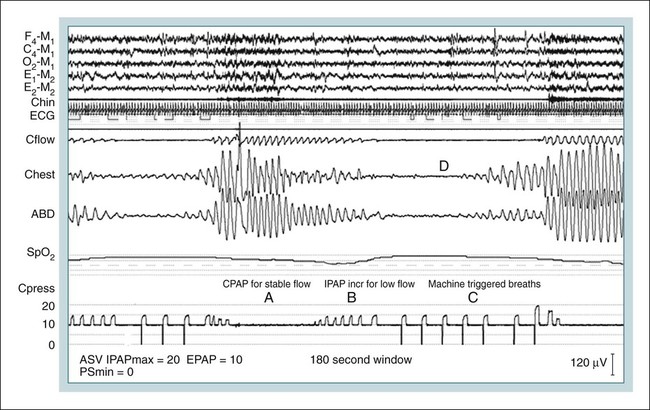
ASV
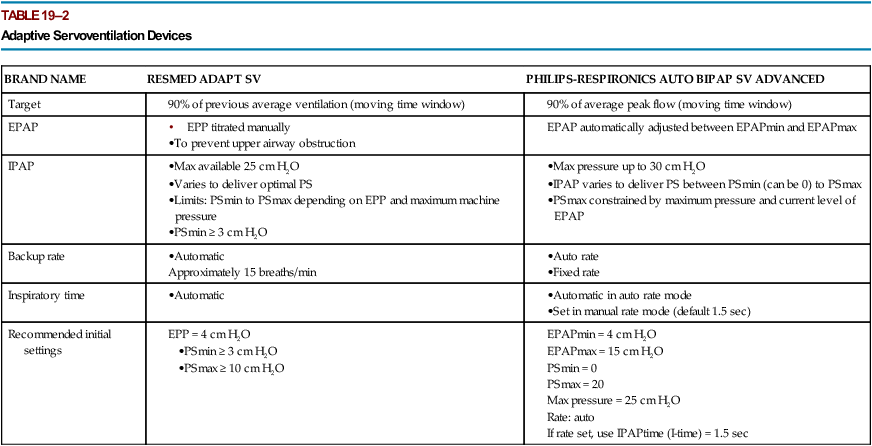
BRAND NAME
RESMED ADAPT SV
PHILIPS-RESPIRONICS AUTO BIPAP SV ADVANCED
Target
EPAP
IPAP
Backup rate
Inspiratory time
Recommended initial settings
Volume-Targeted BPAP
Comfort Measures
Ramp
Flexible Pressure
 hour using a cross-over design.43 A number of subsequent studies in patients on CPAP44–48 or APAP49 have not found an increase in adherence. The mode can still be useful for individual patients who find CPAP difficult to tolerate. Conversely, some patients actually prefer CPAP to flexible PAP.
hour using a cross-over design.43 A number of subsequent studies in patients on CPAP44–48 or APAP49 have not found an increase in adherence. The mode can still be useful for individual patients who find CPAP difficult to tolerate. Conversely, some patients actually prefer CPAP to flexible PAP.
Humidification
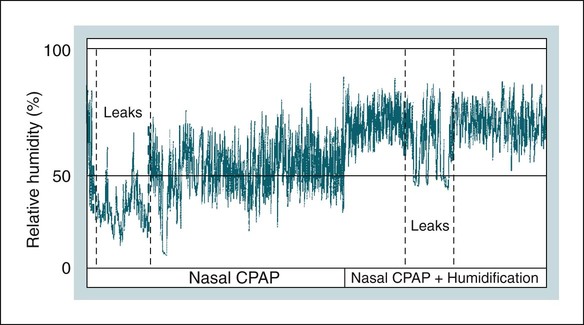
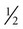 hour greater objective adherence than those on no humidity (Table 19–3). However, several other studies have not found an improvement in adherence56–61 with the use of HH for PAP treatment. Other investigations could not document a benefit from the “prophylactic” use of HH for titration. A criticism of these studies is that patients with baseline nasal congestion or dryness were not targeted. It seems reasonable to use humidity in patients with complaints of nasal congestion or mouth breathing at baseline. Certainly, in some patients, use of HH is crucial, and in others, it may improve satisfaction. Rain-out in the tubing and the mask is a significant problem for some patients. Lowering the CPAP unit to a level below the bed (water flows back into the humidifier chamber by gravity), reducing the humidity setting, or using a tube insulator may help. New technology recently available adjusts the humidifier setting based on room temperature and relative humidity. However, it is not clear that this technology will improve adherence. In the practice parameters for PAP treatment (Appendix 19–1), use of HH is recommended to improve CPAP utilization.3 In the clinical guidelines for titration, having HH available for titration was recommended.62
hour greater objective adherence than those on no humidity (Table 19–3). However, several other studies have not found an improvement in adherence56–61 with the use of HH for PAP treatment. Other investigations could not document a benefit from the “prophylactic” use of HH for titration. A criticism of these studies is that patients with baseline nasal congestion or dryness were not targeted. It seems reasonable to use humidity in patients with complaints of nasal congestion or mouth breathing at baseline. Certainly, in some patients, use of HH is crucial, and in others, it may improve satisfaction. Rain-out in the tubing and the mask is a significant problem for some patients. Lowering the CPAP unit to a level below the bed (water flows back into the humidifier chamber by gravity), reducing the humidity setting, or using a tube insulator may help. New technology recently available adjusts the humidifier setting based on room temperature and relative humidity. However, it is not clear that this technology will improve adherence. In the practice parameters for PAP treatment (Appendix 19–1), use of HH is recommended to improve CPAP utilization.3 In the clinical guidelines for titration, having HH available for titration was recommended.62
HEATED HUMIDITY
COLD PASSOVER HUMIDITY
NO HUMIDITY
Usage (hr/night)
5.52 ± 2.1*
5.15 ± 1.9
4.93 ± 2.2
Epworth Sleepiness Scale
6.2 ± 3.8
7.2 ± 4.8
6.7 ± 3.9
Feeling on awakening
74 ± 15.9†
68.9 ± 23.4
62.0 ± 23.4
Satisfaction with CPAP
73.9 ± 19.1†
72.9 ± 22.6‡
62.3 ± 27.6
Adverse side effects score
4.9 ± 3.3
6.2 ± 3.8
6.5 ± 4.9
Interfaces
Indications for PAP Treatment
Adherence—Definitions and Measurement
PAP Adherence in Large Studies
Factors Influencing Adherence and Importance of Early Adherence
How Much Adherence Is Enough?
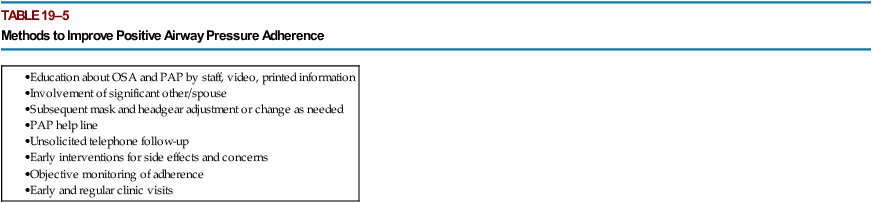
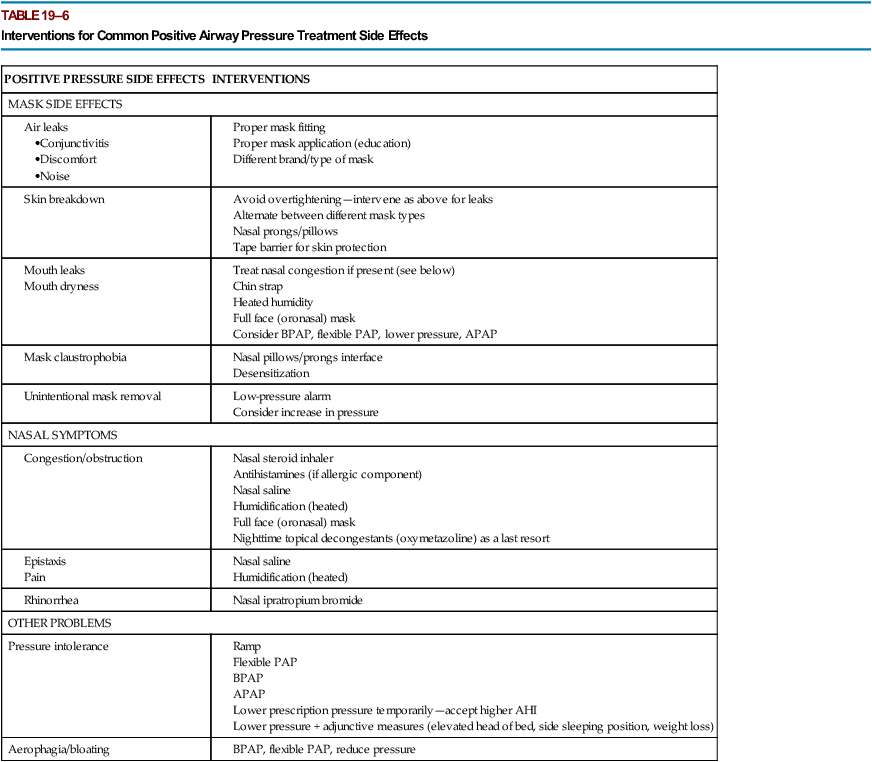
Interventions to Improve Adherence
POSITIVE PRESSURE SIDE EFFECTS
INTERVENTIONS
MASK SIDE EFFECTS
NASAL SYMPTOMS
OTHER PROBLEMS
Pressure intolerance
Aerophagia/bloating
Hypnotics, Alcohol, and CPAP
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neupsy Key
Fastest Neupsy Insight Engine