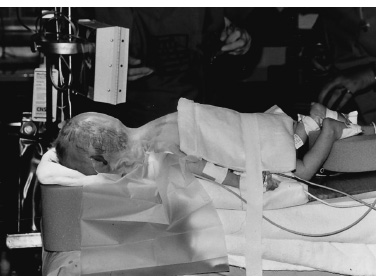
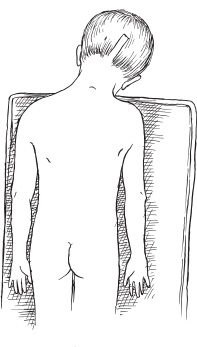
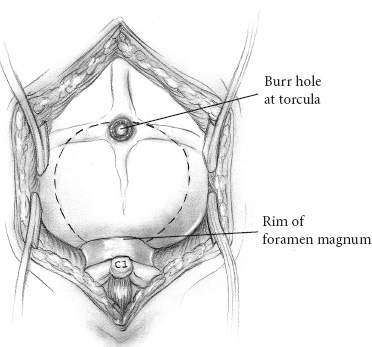
The common posterior fossa tumors in children include medulloblastomas, juvenile pilocytic astrocytomas (JPAs), ependymomas, and brainstem gliomas. Craniotomies are almost always indicated for medulloblastomas, astrocytomas, and ependymomas: to make a tissue diagnosis, to remove tumor and thereby improve prognosis, and to treat the associated hydrocephalus. Craniotomies are probably never indicated for diffuse brainstem gliomas but occasionally are indicated for focal enhancing brainstem gliomas. Corticosteroids are begun at admission to improve symptoms of hydrocephalus and to reduce postoperative edema. Prophylactic anticonvulsants are not needed. Ideally, the scalp is cleansed with an antibacterial shampoo the night before and the morning of craniotomy. If the preoperative scan demonstrates the presence of a posterior fossa tumor with hydrocephalus, hydrocephalus can be treated effectively by an external ventricular drain (EVD). If the child is alert and craniotomy is planned for the next day, we begin corticosteroids and do not insert the EVD until just prior to craniotomy. Precraniotomy shunts almost never are indicated. If the child is drowsy from hydrocephalus and is admitted late in the day, I usually do the EVD and craniotomy that night; however, if additional time is needed to obtain a good-quality scan or a more experienced surgical team in the operating room, it is reasonable to insert the EVD that night and defer the craniotomy until the next day. It is important not to administer preoperative sedatives until shortly before the operation, if at all. After their administration, the child must be monitored closely by pulse oximetry, vital signs, and neurologic checks. A complete description of the anesthetic management during posterior fossa operations can be found in Chapter 69 of the companion textbook, Principles and Practice of Pediatric Neurosurgery. Monitoring for posterior fossa craniotomies includes an electrocardiogram and pulse oximetry, blood pressure monitored by an arterial catheter, and urinary output. Intravenous access at two sites using catheters of 22 gauge or larger (depending on the size of the child) is indicated. It is more important to have two good intravenous catheters than to have central venous access. The utility of Doppler monitoring during posterior fossa craniotomies is debatable. Dopplers frequently detect significant air embolism when craniotomies are performed with the patient in the sitting position, but the use of that position has declined. The likelihood of detecting significant air embolism if the child is in the prone position is small. Because significant air embolism is infrequent if the child is in the prone position I do not routinely use Doppler monitoring. I have not found monitoring of somatosensory-evoked potentials or brainstem-evoked potentials to be helpful and infrequently use either. I prefer to insert EVDs in the right frontal region at the junction of the pupillary line and the coronal suture. I prefer the frontal site because the anatomic coordinates used during insertion of the catheter allow reliable placement of the catheter in the frontal horn of the ventricle and, to a lesser extent, because the frontal position of the catheter postoperatively is more comfortable for children than are posterior catheters. I make a 5- to 7-mm linear incision, perforate the skull with the M8 bit on a Midas Rex drill (Medtronic, Fort Worth, TX), coagulate and open the dura, and then insert a long (30 cm) EVD catheter for the appropriate length into the lateral ventricle. That length, usually 5.5 to 6.5cm, can be determined from measurements made from preoperative scans. The distal end of the catheter is tunneled several centimeters inferiorly toward the temporal region where it exits and is connected to a closed drainage system. I remove 10 to 20 mL of cerebrospinal fluid (CSF) at this time and then close the system until the craniotomy is being performed. Alternatively, some neurosurgeons prefer to insert the catheter posteriorly, either through an occipital burr hole 6 cm above the inion and 2.5 cm lateral to the midline, with a trajectory toward the ipsilateral medial canthus, made after the child is in the prone position, or through a burr hole made in the parietal region at Keen’s point. In my experience, catheters inserted at Keen’s point have not been as well positioned in the frontal horn as have catheters inserted frontally, and catheters inserted occipi-tally have been uncomfortable postoperatively as the child lies supine. In addition, there may be more difficulties cannulating the occipital horn than the frontal horn. A few neurosurgeons do not insert EVDs before removing posterior fossa tumors but prefer to make an occipital burr hole so that the ventricle can be cannulated if the child develops acute hydrocephalus postoperatively. That technique does not allow CSF drainage during the operation, and it assumes that if acute hydrocephalus develops, the ventricle can be cannulated under emergent conditions. I prefer the more controlled conditions that the EVDs provide. The head of an infant 1 year of age or younger usually is stabilized on a large foam block (Fig. 13–1) or on a pediatric horseshoe headholder, ideally padded with gel. For children 1 to 2 years of age, I use either the horseshoe headholder or pediatric pins in the Mayfield holder and I use adult-sized pins in children 4 years of age and thereafter. It is important to remember that not all 1- to 2-year-old children have skulls thick enough to hold pins and that the skull of a child may be thinned by chronic hydrocephalus; in such patients, less pin pressure may be tolerated before the pin causes a focal skull indentation or depressed fracture. Pin pressures of 20 to 30 pounds are used in children 1 to 3 years of age and increased progressively thereafter to the 60-pound pressures used for an adult skull when the child is 12 years of age or older. FIGURE 13–1. Infant positioned prone on foam blocks prior to posterior fossa tumor removal. Once the child is in the prone position, I move him or her to be at the edge of the operating table on my side, and then I angulate the head approximately 30 degrees away from me and flex the neck about 30 degrees (Fig. 13–2). After the head position is finalized, it is important to auscultate the chest to ensure that the neck flexion did not advance the endotracheal tube into the right mainstem bronchus. Once the patient is in the correct position, the surgeon can stand comfortably by the side of the table without leaning over it, and his or her right hand can be held comfortably at the surgical site for several hours if need be, with the assistant standing to the left. Alternatively, some surgeons prefer to keep the child in the center of the operating table and use the quadrascope so that the primary surgeon on one side of the table and the assistant on the opposite side have approximately equal access to the child. The sitting position for craniotomies was used commonly until the past decade, but its disadvantages of air embolism, hypotension, and fatigue of the surgeon’s arms outweigh the advantages of better blood and CSF drainage, and its use has declined substantially. No longer does scalp preparation involve shaving the entire head. Rather, a strip of scalp 5 to 7mm wide can be trimmed along the line of incision with scalp clippers, thereby decreasing the potential of staphylococcal infection, which has been reported after shaving. The operative site is prepared with an iodine-containing solution and alcohol. The solution, Hibiclens, carries a label stating that it is not to be used around the scalp or face because of the risk of conjunctivitis if the solution enters the eye. Exposure of midline tumors begins with a midline incision from the inion to C-2. I control subcutaneous bleeding with the coagulating needle-tip cautery; alternatively, clips can be applied to the skin edges. The cautery can be used to dissect periosteum off the occipital bone laterally and down to the foramen magnum, and then to dissect soft tissues away from C-1 and the space between the foramen magnum and C-1. As the periosteum is dissected off the occipital bone, veins often are divided where they enter the bone and can bleed substantially. Their bleeding can be controlled by electrocautery or bone wax. If a Doppler is used and air embolism is detected by its characteristic washing-machine sound, treatment includes flooding the operative field with saline, increasing the venous pressure by lowering the head of the bed or compressing the jugular veins, and aspirating air from a central venous catheter if one is available. Subcutaneous tissues are held apart with cerebellar retractors. Pediatric neurosurgeons increasingly perform an occipital craniotomy rather than a craniectomy, which leaves a concave depression in the skull and a potential site for trauma. For a craniotomy, a single burr hole can be made in the midline at approximately the level of the torcula (obviously taking considerable care not to enter it), or two burr holes can be made on either side of the midline in the region of the transverse sinus (Fig. 13–3). These burr holes can be made safely with an M8 bit on a Midas Rex drill; I have reservations about making burr holes over the torcula with powered burr-hole makers. The traditional exposure is by an occipital craniectomy, removing bone with Kerrison and Leksell rongeurs from the foramen magnum upward until the inferior aspect of the transverse sinus is exposed.
PREOPERATIVE CARE
INTRAOPERATIVE TECHNIQUES
Monitoring
External Ventricular Drains
Positioning
Scalp Preparation
Exposure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


