22
Posterior Interhemispheric Approach to Pineal Region and Brainstem
In the United States, pineal-region tumors constitute approximately 1 to 3% of the intracranial masses.1 Victor Horsley attempted the first direct removal of a pineal tumor in 1905.2 This was the first attempt to remove any lesion in the region of the posterior third ventricle recorded in the modern era. Cushing3 proposed the idea of surgical treatment of pineal tumors but viewed this region as surgically inaccessible. In 1926, Krause4 reported three patients with a pineal tumor on whom he operated using a posterior fossa approach above the cerebellum (infratentorial, supracerebellar) with the patient in a sitting position.
In 1931, W.P. Van Wagenen5 proposed the transcortical transventricular approach, which never gained wide acceptance due to the difficulties associated with this approach. Bennet Stein6,7 later improved upon the approach proposed by Krause, using microsurgical techniques and reported several series on his improved infratentorial, supracerebellar approach with the patient in the sitting position.
Walter Dandy8 was accredited with the first scholarly analysis with his supratentorial parafalcine approach. He started this approach after an initial trial on animals. He placed the patient in either a semi-sitting position or semiprone position with the side of the approach uppermost, thus retracting the occipital lobe against the gravity. He dissected down the medial side of the occipital lobe. On reaching the tentorial incisura, he sectioned the splenium of the corpus callosum and dissected the venous confluence.
Technical variations for similar lesions include the occipital transtentorial approach described by James Poppen9 in 1966, and the combined supratentorial and infratentorial transsinus approach described by Ziyal et al.10
Not only have the surgical approaches evolved, but also advances in the neuroimaging, endoscopic techniques, and neuronavigation have greatly helped in the successful management of the pineal tumors with an associated decline in mortality.
♦ Posterior Approaches to the Pineal Region
Posterior approaches to pineal tumors can be divided into supratentorial and infratentorial. Supratentorial approaches include the occipital transtentorial,9 interhemispheric transcallosal,11 and interhemispheric retrocallosal.12 Infratentorial approaches include the infratentorial supracerebellar4 and infratentorial paramedian supracerebellar.13
Two approaches most commonly performed for pineal region tumors are the occipital interhemispheric transtentorial and infratentorial supracerebellar. The wide variety of surgical positions applied to reach this location reflect the difficulties in accessing the lesions. The positions utilized for these approaches vary from prone to sitting to semiprone, semi-sitting, three-quarter prone/park-bench, and Concorde. The availability of newer imaging techniques and intraoperative neuronavigation methods have further improved the outlook and the operative results remarkably. Studies have reported minimal to zero mortality.14–17 Intraoperative image-guided surgery has been utilized as a routine at present in our experience.
♦ Indications
Traditionally the posterior interhemispheric approach was applied to access tumors located at the posterior third ventricle, especially pineal tumors growing superiorly or laterally to the trigone of the lateral ventricle, or tumors growing into the ambient cistern. This approach was also used for tumors above the venous structures, around the vein of Galen.16,18,19 Tumors arising in the splenium of the corpus callosum or the medial occipital lobe are easily removed via this route. Extended indications include tumors arising from the brainstem, vascular malformations, or aneurysms of the P2/3 segment of the posterior cerebral artery. Other extended indications include vascular malformations of the vein of Galen and lesions of the superior vermian/cerebellar area and cavernomas of the dorsal midbrain.20–28
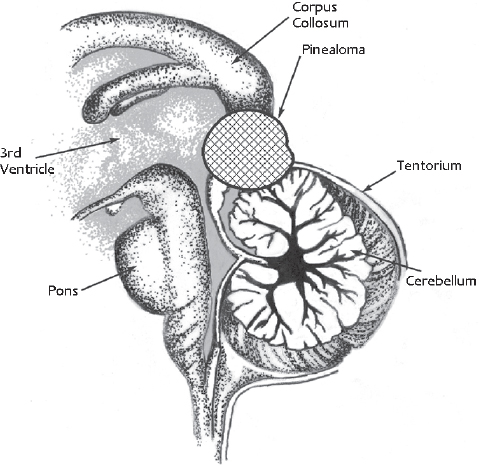
Fig. 22.1 A standard pineal region tumor can be directly accessed by the occipital interhemispheric approach. This does not require sectioning of the tentorium or resection of the splenium of the corpus callosum.
A lesion in the pineal gland location is easily accessed by the occipital interhemispheric approach, without resection of the splenium or sectioning the tentorium (Fig. 22.1). Tumors reaching anterior and superior to the pineal region might have an overhanging splenium and occasionally require dissection or resection of part of the splenium (Fig. 22.2). Massive pineal region tumors, such as a large meningioma, pinealoblastoma, or tumors arising in the superior surface of the cerebellar vermis, require sectioning of the tentorium cerebelli, to facilitate complete access to these lesions (Fig. 22.3). It is noteworthy that most of the lesions arising from the cerebellar vermis are supplied by the superior cerebellar artery (SCA). Kurokawa et al23 suggested that the superior cerebellar artery is a major feeding artery of these tumors. Another advantage of this approach is that it enables the surgeon to gain access to the SCA early in the procedure.
♦ Preoperative Evaluation
Apart from routine evaluation of the patient for fitness for anesthesia, the surgical workup includes good-quality multiplanar magnetic resonance imaging (MRI) studies with and without contrast for precise localization of the tumor.6 Angiography may be beneficial in selected instances, such as vascular pathologies and selected vascular tumors. In these instances, an angiogram is helpful in delineating important surrounding vascular structures.29 MRI is mostly adequate for the diagnosis of cavernous angiomas of the brainstem as well as posterior third ventricular region tumors. MR spectroscopy may help in preoperative histologic diagnosis. A standard digital subtraction angiography (DSA) for vascular malformations and aneurysms provides the location and helps in surgical strategies. It is important to note that a vascular nidus located lateral to the P2/P3 segments of the posterior cerebral artery (PCA) on the anteroposterior angiographic views may not be a suitable pathology to approach using the interhemispheric approach. This would necessitate severe occipital lobe retraction to reach the nidus, and it can produce irreversible hemianopsia.20
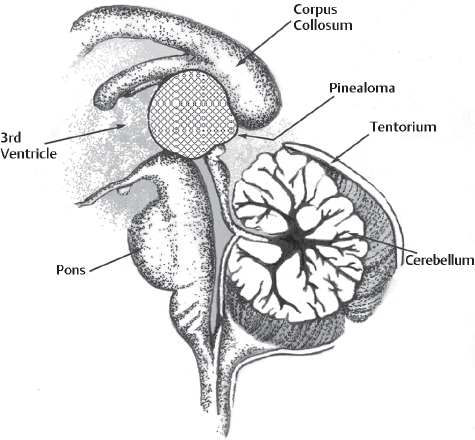
Fig. 22.2 Lesions growing anteriorly and superiorly reaching under the splenium of the corpus callosum or infiltrating it would require resection of the corpus callosum. Sometimes endoscopic application is useful in avoiding the resection of splenium. Similarly, tumors that can be sucked out, such as those in the dermoid and epidermoid, can be removed without causing damage to splenium.
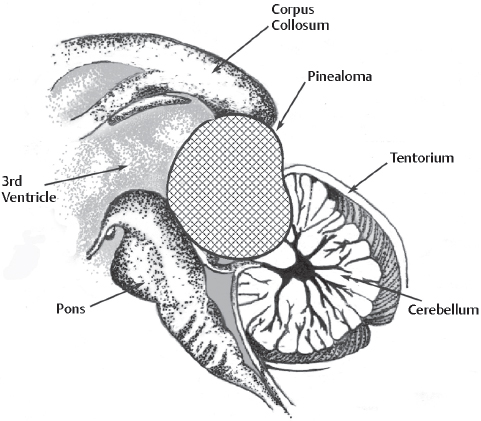
Fig. 22.3 Large tumors like meningiomas or pinealoblastoma or a low-level posterior cerebral artery (PCA) aneurysms and cerebellar vermian tumor are better managed by opening the tentorium cerebelli.
If the radiologic studies are suggestive of a germ cell tumor, then an MRI scan of the whole craniospinal axis is indicated. In these instances, measurement of tumor markers, by using, for example, humor chorionic gonadotropin (hCG), carcinoembryonic antigen (CEA), or α-fetoprotein, is needed.1 The distinction between germinomatous and nongerminomatous tumors affects the patient’s management. Germinomatous tumors show good response to gamma knife radiosurgery.30 Nongerminomatous germ cell tumors are resistant to radiotherapy, but may respond to chemotherapy.30a,30b
♦ Positioning of the Patient
Various positions have been described for this approach, as the semi-sitting position described by Yasargil et al,26 and also, the Concorde position. The lateral position has been found to be suitable in our experience.17 Significant air embolism might result from the sitting or semi-sitting positions, which sometimes produces clinically relevant changes.20
In the operating suite, the endotracheal tube is placed with the patient in the supine position; a central venous catheter and an arterial line are also placed. Following lumbar drain placement, the patient is then placed in the lateral position with the head turned 30 degrees toward the floor (which places the side of access in the dependent position), allowing the occipital lobe to fall inferiorly, to facilitate minimal retraction. A beanbag in conjunction with cloth tape and padding secures and maintains the body position; the superior shoulder is gently pulled using a strip of tape with foam under it, attached to the foot of the bed. The lower knee has to be bent with foam padding placed between the knees and malleoli, under the axilla, and around the elbows. After placement of the head in a Mayfield head-holder and subsequent fixation to the bed, image registration using fiducial markers and the Stealth neuronavigation system (Medtronic Sofamor Danek, Memphis, TN) performed.
♦ Operative Technique
Frameless stereotaxy helps in planning the optimal skin incision, in relation to the underlying superior sagittal sinus, as well as in determining the optimal trajectory (avoiding apparent bridging veins and the shortest distance to the lesion). A very thin strip of hair (approximately 2 cm wide) is shaved, and then a horseshoe-shaped incision is placed on the side of the craniotomy, in the occipital region. The base of the skin flap goes along the superior nuchal line, and the medial limb of the incision is just past the midline. The skin flap is elevated along with the galea and pericranium, and reflected inferiorly. A single bur hole is made near the midline, and a rectangular bone flap (approximately 5 cm at midline × 3 cm) is elevated using a high-speed drill. The edge of the superior sagittal sinus is barely visualized. The dural incision is made utilizing the entire space of the craniotomy and turned medially with the base on the sinus (Fig. 22.4). The utmost care is required to avoid damage to the sinus. Bridging veins are scarce in the occipital region, and those present can be easily avoided.31 Having the occipital lobe in a dependent position facilitates a surgical corridor, which requires minimal arachnoidal dissection to visualize the white posterior corpus callosum, not to be confused with the yellow cingulated gyrus, which has a resemblance to the corpus callosum (Fig. 22.5A). Opening the lumbar drain at this time also facilitates brain relaxation. At this stage particularly, we have found it very useful to confirm our position and trajectory using frameless stereotaxy, and alter it accordingly to provide optimal access. The posterior end of the tentorial notch is typically encountered next, where the falx cerebri joins with the tentorium cerebelli. A thick layer of arachnoid, forming the posterior boundary of the quadrigeminal cistern, is now sharply incised to enter the quadrigeminal cistern.
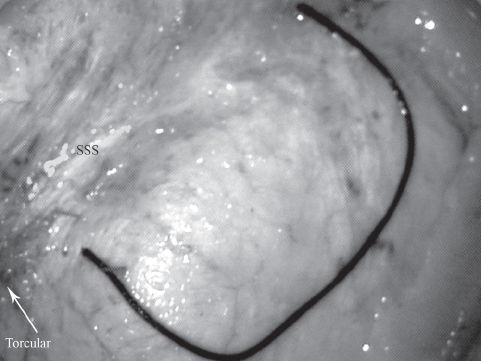
Fig. 22.4 A dural incision is shown in a cadaveric study. Note that the craniotomy crosses the superior sagittal sinus (SSS), and that the dural flap is cut up to the level of the sinus, so that exposure can be maximized.
Surgical Procedure to Remove the Lesion
The major venous network (veins associated with the vein of Galen) is usually seen covering a tumor in the pineal region. Although some authors believe that this is problematic by presenting a barrier between the surgeon and the lesion, we have found that direct visualization of the venous structures at this early stage of dissection actually aided us in protecting the venous structures (Fig. 22.5B). Using meticulous, sharp dissection, these structures can be gently separated and retracted to provide direct access to the lesion.
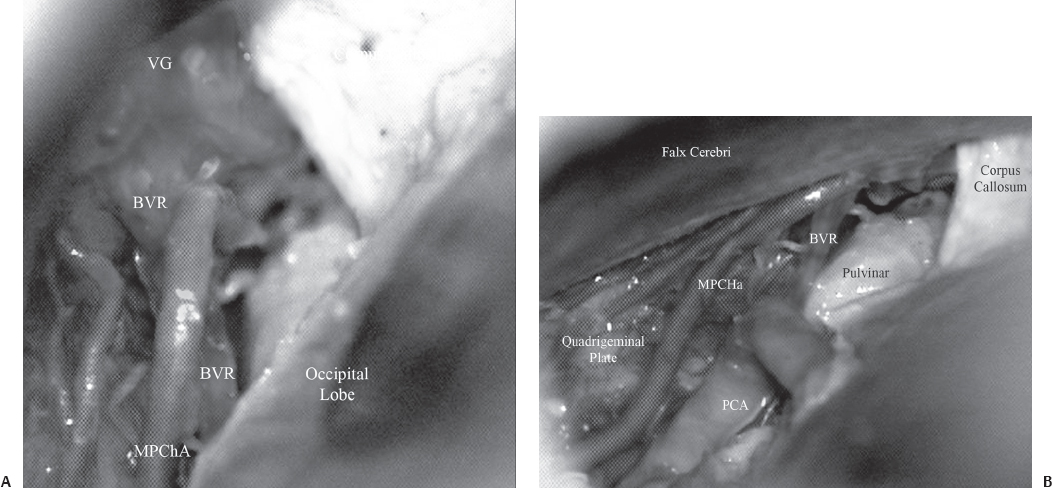
Fig. 22.5 Anatomy of the posterior third ventricular region. (A) Just below the corpus callosum, the vein of Galen (VG) is seen. The basal vein of Rosenthal (BVR) is seen joining the vein of Galen. The medial posterior choroidal artery (MPChA) is also seen. (B) On retracting the occipital lobe laterally, the pulvinar of the thalamus is seen. The medial posterior choroidal artery is seen to enter the velum interpositum. The posterior cerebral artery is seen ascending up along the medial surface of the occipital lobe. PCA, posterior cerebral artery.
Different lesions, depending on the size, gross consistency, origin, and location in relation to the venous structures, can be resected using different strategies. For example, in tentorial meningiomas, the venous system is usually largely displaced ventrally, and the tumor is encountered before these veins; thus tumors are decompressed piecemeal, and the capsule can be removed at the end. Pineal region cysts are punctured and the contents are aspirated or sucked out so that the walls of the cysts collapse and can be excised completely after gentle separation from surrounding structures.
For arteriovenous malformations (AVMs), it is important to mobilize the medial occipital and the deep venous veins to expose the medial surface of the occipital lobe.32 Mobilization of these veins enables the dependent occipital lobe to fall away further. Moreover, this enables the surgeon to visualize the posterior end of choroidal fissure and cisterna ambiens. Also, a clear view of the P2 and P3 segments of the posterior cerebral artery is obtained. By following the branches of the posterior cerebral artery, the nidus of the AVM can be reached. In addition, this exposure also provides entry into the trigone of the lateral ventricle via the choroidal fissure. Anterior dissection, just below the formation of the vein of Galen, reveals a dense network of the arachnoid mater (the velum interpositum cistern), which leads into the third ventricle when divided. Coagulation of the tela choroidea of the third ventricle provides entry into this cavity. Just inferior to this, the pineal gland is visualized, along with the posterior choroidal artery entering the gland. Below the pineal gland, the quadrigeminal plate is seen, and just posterior, the superior vermis of the cerebellum can be visualized.
♦ Case Illustrations
Case 1
A 53-year-old woman presented with recurrent episodes of bleeding from a midbrain cavernoma (Fig. 22.6). She also had one episode of bleeding from a cavernoma in the occipital lobe, and previously underwent multiple operations for cavernomas located elsewhere in the brain. She presented this time with an altered sensorium, oculomotor palsy, and hemiparesis, following a bleed from a midbrain lesion. The patient underwent surgery, using the occipital inter-hemispheric approach (OIHA) with frameless stereotaxy guidance. Neither the tentorium nor the splenium was cut; the lesion was completely resected (Fig. 22.7). The patient’s neurologic deficits gradually resolved completely, and she has returned to normal.
Case 2
A 32-year-old man presented with headache and altered sensorium. Computed tomography (CT) of the head showed a hematoma in the medial occipital lobe. Angiography revealed a medial occipital AVM (Fig. 22.8A,B). The patient was operated on with the occipital interhemispheric approach. The AVM was totally resected (Fig. 22.8C). At 6-year follow-up, he is leading a fully active life, with no apparent new neurologic deficit.
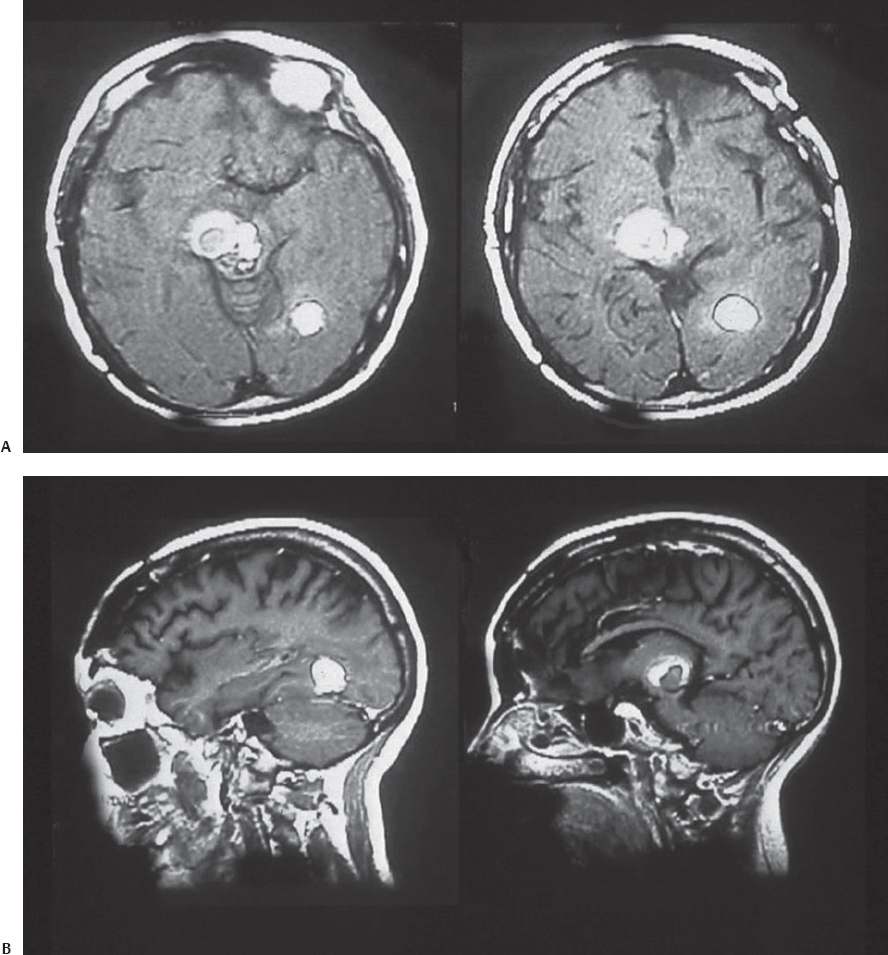
Fig. 22.6 T1 axial (A) and sagittal (B) magnetic resonance imaging (MRI) scans of the patient in Case 1, showing a cavernoma present in the midbrain and occipital lobe.
Case 3
A 77-year-old woman presented with subarachnoid hemorrhage (Hunt-Hess grade I). Angiography revealed a distal posterior cerebral artery aneurysm (P3 segment) and a small AVM in the cerebellum. The distribution of blood and the appearance of the aneurysm suggested that the aneurysm had likely bled. Using OIHA without frameless stereotaxy, finding the aneurysm was considerably difficult. Ultimately, it was visualized after the tentorium was incised; the posterior cerebral artery had a somewhat anomalous course, dipping below the tentorium. The aneurysm was successfully clipped, and the patient had an uneventful recovery.
♦ Modifications
Shirane et al17 utilized the occipital transtentorial approach for pineal region tumors in 31 patients, and the applied navigation system with endoscopy was used in 16 of these patients. All 16 patients had excellent outcomes without any complications. In the other 15 patients neuronavigation could not be used, and complications were seen in five of the 15 patients in the form of seizures and hemianopsia. In contrast, none of the 16 patients who had image-guided surgery and endoscopic assistance developed any new deficits. The authors feel that the use of assisted systems, such as neuronavigation and endoscopy, during microsurgery increases the surgery’s safety and accurate.33–39
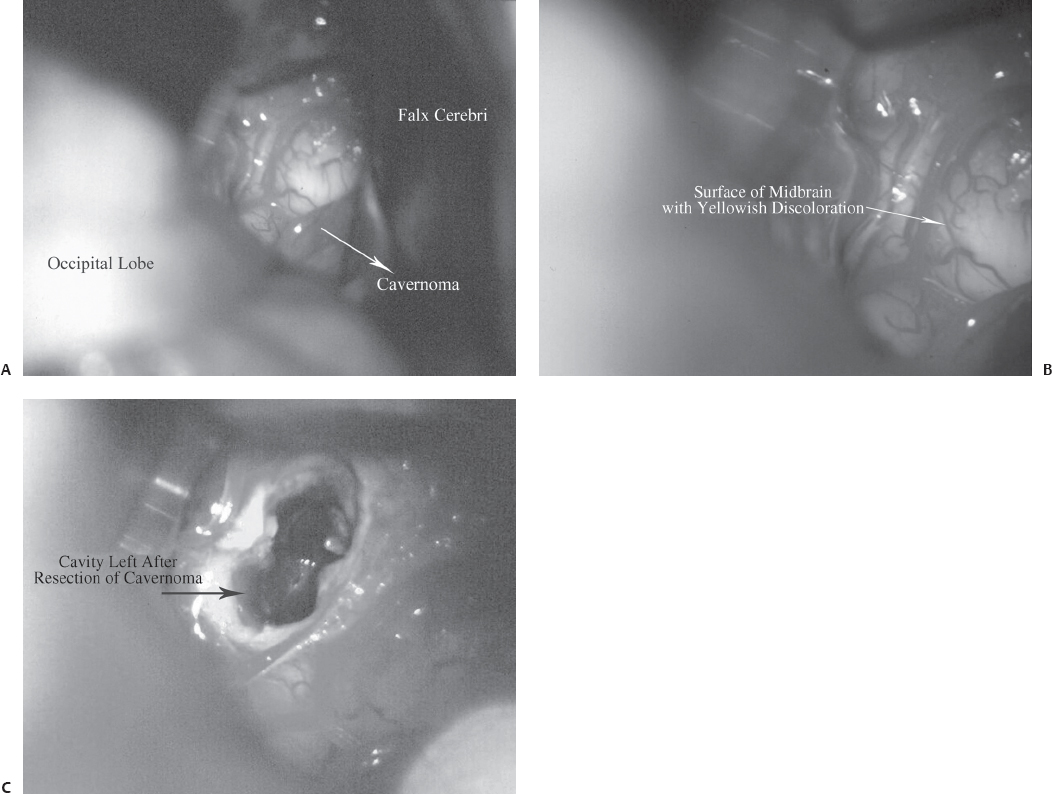
Fig. 22.7 Intraoperative pictures for the patient in Case 1. (A) Exposure of the posterior surface of the midbrain by OIHA. (B) The midbrain surface shows a yellowish tinge, which suggests hemorrhage. (C) Postresection cavity in the midbrain.
Nazzaro et al16 operated on 12 patients, who were placed in the semi-sitting position; none of them had an air embolism or hypotension. However, a detailed neuro-ophthalmology workup was performed in all cases, which revealed a transient visual field defect in all patients. and it persisted in two patients. Lapras et al40 suggested that the incidence of visual field defects may be reduced by retracting the occipital lobe laterally instead of the superolateral lifting of the lobe with the patient in the sitting or semi-sitting position.
Konovalov et al29 placed patients in the semi-sitting or three-quarter positions when operating on meningiomas of the pineal region, and performed a craniotomy from the nondominant side. An angiographic evaluation was obtained in seven of 10 patients for topography of the blood vessels around the tumor, especially the great vein of Galen and the straight sinus. According to the authors, the three-quarter prone position provides better visualization and less retraction of occipital lobe, albeit with unfamiliar anatomic detail.
Yasargil41 proposed the occipital interhemispheric approach as the first choice for aneurysms arising from the P3 segment of the posterior cerebral artery. The selection of the approach for the P2 segment, however, depends on the surgeon’s choice. The P2p segment is located between the cerebral peduncle and the quadrigeminal cistern,42 and it traverses the ambient cistern. Terasaka et al24 found that this segment in the ambient cistern is more easily reached by the occipital interhemispheric approach than by the subtemporal or pterional transsylvian approach. Based on their cadaver study and clinical experience, these authors suggest that high P2p segment aneurysms are better approached using this approach, especially when an angiogram in the lateral view shows the aneurysm to be close to the anterior choroidal artery in the temporal horn. However, just as in cases in which the vein of Labbé restricts temporal lobe retraction in the subtemporal approach, the occipital interhemispheric route also would face limitations due to the internal occipital vein, especially when the parent artery is located behind the aneurysm. These authors also believe that vascular reconstruction involving the PCA would be nearly impossible. Touho et al25 reported on an anastomosis of the occipital artery to the PCA, with interposition of a graft from the superficial temporal artery for severe stenosis of the distal basilar artery, with a good outcome. They propose this approach as a safer one compared with other routes utilized for an anastomosis between the superficial temporal artery and the superior cerebellar artery or the PCA. Most of these complications are attributed to severe temporal lobe retraction.43,44
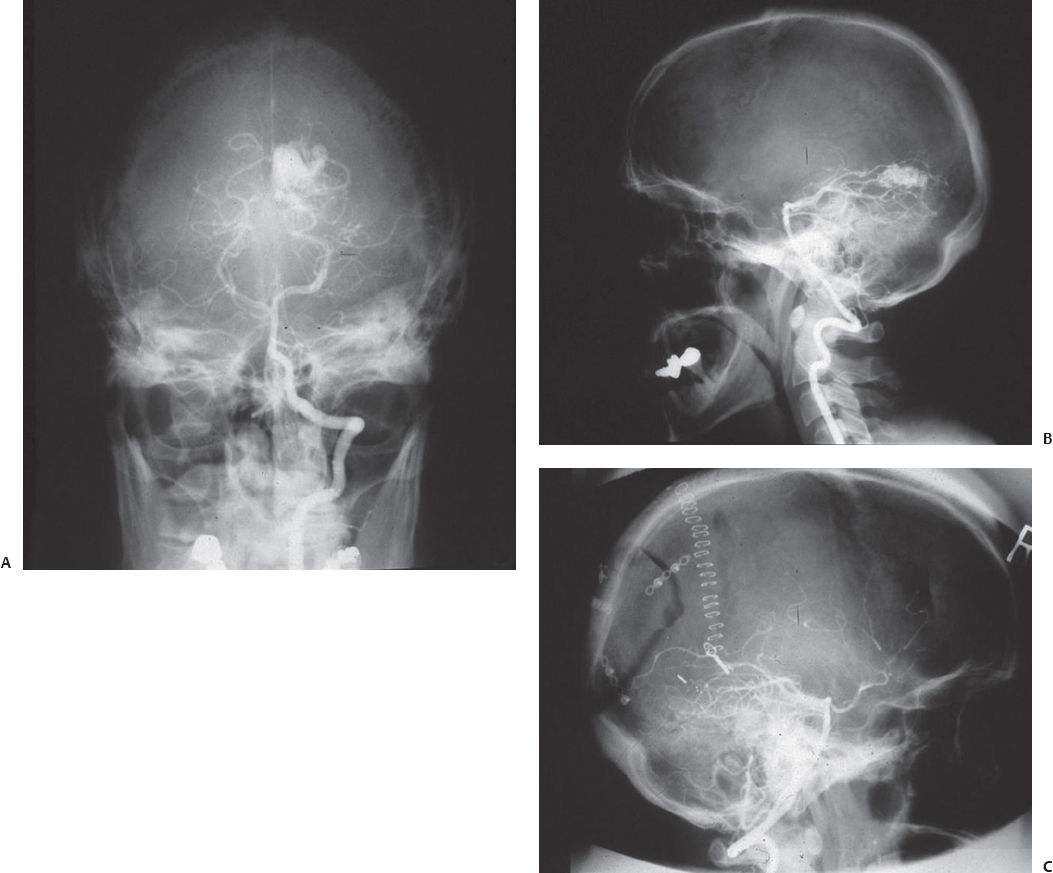
Fig. 22.8 Intraoperative (A,B) and postoperative (C) angiograms of the patient in Case 2, who had a medial occipital arteriovenous malformation.
Ausman et al45 suggested the possibility of reaching the cerebellar vermis from above by cutting the tentorium via the occipital interhemispheric approach. The possibility of reaching the cerebellum by this route is especially interesting because the vascular supply of lesions located in this part of the cerebellum comes from the superior cerebellar artery, and this artery is visualized at the initial stages of surgery when the tentorium is incised from above. Kurokawa23 et al reported a series of six cases with cerebellar vermian tumors excised via the occipital interhemispheric transtentorial approach. A preliminary report of the transtentorial visualization of the cerebellar region appears in James Poppen’s9 report. We have utilized the transtentorial access to reach the P2/3 segment of the PCA with an aneurysm. Kurokawa et al found easy access to unilateral lesions of the cerebellar vermis using this midsagittal plane approach. Up to 20 mm of the opposite vermian surface could be easily reached from the midline owing to the wider operative field afforded by this approach. Unusual bleeding sometimes comes from the cut edge of the tentorium that can be easily controlled by hemoclip application.
In the recent literature, endoscopic assistance has been found to be useful in visualization of the deep-seated lesions and surrounding structures while brain retraction is minimized.17,46 Morbidity and mortality following microsurgical approaches using the navigation system and endoscopic assistance have been reduced considerably.47,48 An additional advantage of utilizing an endoscope is the feasibility of performing a third ventriculostomy in cases of malignant tumors with infiltration of the surrounding structures.49 Endoscopy is sometimes complementary to neuronavigation to compensate for possible brain shifts.
♦ Complications
The main surgical complications following this procedure are hemorrhage, brainstem damage, and visual field defects. Intra- and postoperative hemorrhages originate mainly from the veins around the tumor, the most difficult task being separation of the internal cerebral vein from the tumor. A small portion of the splenium may require excision to expose the internal cerebral vein safely and to preserve it. The Valsalva maneuver at the end of tumor excision confirms complete hemostasis.
Neural structures and the brainstem may be damaged by direct surgical trauma or due to interrupted vascular supply. Fukui et al50 reported central hearing loss following surgery, which was attributed to damage to the auditory fibers from the inferior colliculus to the medial geniculate body, passing across the dorsolateral surface of the midbrain. Obstruction of the medial posterior choroidal artery may cause ischemia to the medial thalamus.
The occipital interhemispheric approach has the potential to damage the occipital lobe, caused by retraction. The internal occipital vein usually originates from the inferior medial surface of the occipital lobe and runs anteromedially toward the pineal region, entering the vein of Galen. Injury to this vein often results in either transient or permanent hemianopia.16,51 Postoperative ocular deficits may also appear following injury to the tectum when using this approach.40,52,53
Usually, no morbidity follows sacrifice of the occipital veins, draining to the torcular Herophili or the transverse sinus.40,54 Division of the precentral vein usually does not cause morbidity either.55 It has been our experience, as well as that of others, that a gravity-assisted occipital lobe retraction helps reduce these complications.31,45,52,56,57
Several authors reported zero mortality with this approach.14,16,17,29,49,52 A 3 to 10% mortality has been reported by Neuwelt et al,54 Luo et al,56 and Ventureyra.31 Air embolism and hypotension have not been recorded as significant intraoperative problems when using the sitting or semi-sitting position.16,29
♦ Conclusion
Different approaches to the posterior third ventricular and pineal regions have been described. The key points in the application of the occipital interhemispheric approach are as follows: (1) Surgeons must be familiar with patient positioning, which can provide brain retraction by gravity. Surgeons choose from the various available surgical positions, depending on their experience. Appropriate positioning and orientation to the anatomic detail not only improve the surgical corridor but also reduce the retraction on the occipital lobe and the complications associated with it. (2) Preoperative planning includes the acquisition of a neuronavigational scan, using posterior fiducial markers to achieve greater accuracy. (3) Neuronavigation is important in the following situations: (a) in planning the skin incision and trajectory (to allow the shortest trajectory while avoiding apparent bridging veins); (b) while traversing the corridor between the occipital lobe and falx, to ensure the trajectory toward the target; (c) once near the target, in determining the shortest and safest route of entry toward an intraparenchymal lesion; and (d) in defining the extent of the resection at the end. In the event of a brain shift following opening, the landmarks on the navigation system will assist in providing accuracy during the procedure.
References
1. Kyritsis AP. Management of primary intracranial germ cell tumors. J Neurooncol 2010;96:143–149 PubMed
2. Little KM, Friedman AH, Fukushima T. Surgical approaches to pineal region tumors. J Neurooncol 2001;54:287–299 PubMed
3. Cushing H. Intracranial tumors: Notes Upon a Series of Two Thousand Verified Cases with Surgical-Mortality Percentages Pertaining Thereto. Springfield, IL: Charles C. Thomas, 1932:150
4. Krause F. Operative Frielegung der Vierhugel, nebst Beobachtungen uber Hirndruck und Dekompression. Zentralbl Chir 1926;53:2812–2819
5. Van Wagenen WP. A surgical approach for the removal of certain pineal tumors: report of a case. Surg Gynecol Obstet 1931;53:216–220
6. Bruce JN, Stein BM. Surgical management of pineal region tumors. Acta Neurochir (Wien) 1995;134:130–135 PubMed
7. Stein BM. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg 1971;35:197–202 PubMed
8. Dandy WE. Operative experience in cases of pineal tumor. Arch Surg 1936;33:19–46
9. Poppen JL. The right occipital approach to a pinealoma. J Neurosurg 1966;25:706–710 PubMed
10. Ziyal IM, Sekhar LN, Salas E, Olan WJ. Combined supra/infratentorial-transsinus approach to large pineal region tumors. J Neurosurg 1998; 88:1050–1057 PubMed
11. Dandy W. An operation for the removal of pineal tumors. Surg Gynecol Obstet 1921;33:113–119
12. Glasauer FE. An operative approach to pineal tumors. Acta Neurochir (Wien) 1970;22:177–180 PubMed
13. Yasargil M. Paramedian supracerebellar approach. In: Yasargil M, ed. Microneurosurgery, Vol 1. New York: Georg Thieme Verlag, 1984:242
14. Cho BK, Wang KC, Nam DH, et al. Pineal tumors: experience with 48 cases over 10 years. Childs Nerv Syst 1998;14:53–58 PubMed
15. Edwards MS, Hudgins RJ, Wilson CB, Levin VA, Wara WM. Pineal region tumors in children. J Neurosurg 1988;68:689–697 PubMed
16. Nazzaro JM, Shults WT, Neuwelt EA. Neuro-ophthalmological function of patients with pineal region tumors approached transtentorially in the semisitting position. J Neurosurg 1992;76:746–751 PubMed
17. Shirane R, Shamoto H, Umezawa K, et al. Surgical treatment of pineal region tumours through the occipital transtentorial approach: evaluation of the effectiveness of intra-operative micro-endoscopy combined with neuronavigation. Acta Neurochir (Wien) 1999;141:801–808, discussion 808–809 PubMed
18. Konovalov AN, Pitskhelauri DI. Principles of treatment of the pineal region tumors. Surg Neurol 2003;59:250–268 PubMed
19. Lozier AP, Bruce JN. Meningiomas of the velum interpositum: surgical considerations. Neurosurg Focus 2003;15:E11 PubMed
20. Batjer H, Samson D. Surgical approaches to trigonal arteriovenous malformations. J Neurosurg 1987;67:511–517 PubMed
21. Chanda A, Nanda A. Multiple cavernomas of brain presenting with simultaneous hemorrhage in two lesions: a case report. Surg Neurol 2002;57:340–344, discussion 334–335 PubMed
22. Hernesniemi J. Arteriovenous malformations of the vein of Galen: report of three microsurgically treated cases. Surg Neurol 1991;36:465– 469 PubMed
23. Kurokawa Y, Uede T, Hashi K. Operative approach to mediosuperior cerebellar tumors: occipital interhemispheric transtentorial approach. Surg Neurol 1999;51:421–425 PubMed
24. Terasaka S, Sawamura Y, Kamiyama H, Fukushima T. Surgical approaches for the treatment of aneurysms on the P2 segment of the posterior cerebral artery. Neurosurgery 2000;47:359–364, discussion 364–366 PubMed
25. Touho H, Karasawa J, Ohnishi H, Kobitsu K. Anastomosis of occipital artery to posterior cerebral artery with interposition of superficial temporal artery using occipital interhemispheric transtentorial approach: case report. Surg Neurol 1995;44:245–249, discussion 249–250 PubMed
26. Ya argil MG, Jain KK, Antic J, Laciga R. Arteriovenous malformations of the splenium of the corpus callosum: microsurgical treatment. Surg Neurol 1976;5:5–14 PubMed
argil MG, Jain KK, Antic J, Laciga R. Arteriovenous malformations of the splenium of the corpus callosum: microsurgical treatment. Surg Neurol 1976;5:5–14 PubMed
27. Shirane R, Kumabe T, Yoshida Y, et al. Surgical treatment of posterior fossa tumors via the occipital transtentorial approach: evaluation of operative safety and results in 14 patients with anterosuperior cerebellar tumors. J Neurosurg 2001;94:927–935 PubMed
28. Santi L, Tomita T. The occipital transtentorial approach for cerebellar arteriovenous malformation in a child. Childs Nerv Syst 2000;16:129– 133 PubMed
29. Konovalov AN, Spallone A, Pitzkhelauri DI. Meningioma of the pineal region: a surgical series of 10 cases. J Neurosurg 1996;85:586–590 PubMed
30a. Wenger M, Lövblad KO, Markwalder R, Taub E. Late recurrence of pineal germinoma. Surg Neurol 2002;57:34–39, discussion 39–40 PubMed
30b. Balmaceda C, Heller G, Rosenblum M, et al. Chemotherapy without irradiation—a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol 1996;14:2908–2915
31. Ventureyra EC. Pineal region: surgical management of tumours and vascular malformations. Surg Neurol 1981;16:77–84 PubMed
32. Pickard JD. Advances and technical standards in neurosurgery. In: Krayenbühl H, ed. Vol 27. New York: Springer-Verlag.; 2002:230–231
33. Yamini B, Refai D, Rubin CM, Frim DM. Initial endoscopic management of pineal region tumors and associated hydrocephalus: clinical series and literature review. J Neurosurg 2004;100(5 Suppl Pediatrics):437– 441
34. Oi S, Shibata M, Tominaga J, et al. Efficacy of neuroendoscopic procedures in minimally invasive preferential management of pineal region tumors: a prospective study. J Neurosurg 2000;93:245–253 PubMed
35. Gangemi M, Maiuri F, Colella G, Buonamassa S. Endoscopic surgery for pineal region tumors. Minim Invasive Neurosurg 2001;44:70–73 PubMed
36. Chernov MF, Kamikawa S, Yamane F, Ishihara S, Kubo O, Hori T. Neurofiberscopic biopsy of tumors of the pineal region and posterior third ventricle: indications, technique, complications, and results. Neurosurgery 2006;59:267–277, discussion 267–277 PubMed
37. Youssef AS, Keller JT, van Loveren HR. Novel application of computer-assisted cisternal endoscopy for the biopsy of pineal region tumors: cadaveric study. Acta Neurochir (Wien) 2007;149:399–406 PubMed
38. Schroeder HW, Wagner W, Tschiltschke W, Gaab MR. Frameless neuronavigation in intracranial endoscopic neurosurgery. J Neurosurg 2001; 94:72–79 PubMed
39. Kim IY, Jung S, Moon KS, Jung TY, Kang SS. Neuronavigation-guided endoscopic surgery for pineal tumors with hydrocephalus. Minim Invasive Neurosurg 2004;47:365–368 PubMed
40. Lapras C, Patet JD, Mottolese C, Lapras C Jr. Direct surgery for pineal tumors: occipital-transtentorial approach. Prog Exp Tumor Res 1987; 30:268–280 PubMed
41. Yasargil MG. Vertebrobasilar aneurysms. In: Yasargil MG, ed. Microneurosurgery: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results, Vol 2. Stuttgart: Georg Thieme, 1984:260–269
42. Zeal AA, Rhoton AL Jr. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg 1978;48:534–559 PubMed
43. Ausman JI, Diaz FG, Mullan S, Gehring R, Sadasivan B, Dujovny M. Posterior inferior to posterior inferior cerebellar artery anastomosis combined with trapping for vertebral artery aneurysm. Case report. J Neurosurg 1990;73:462–465 PubMed
44. Dempsey RJ, Hopkins J, Bittman EL, Kindt GW. Total pinealectomy by an occipital parasagittal approach in sheep. Surg Neurol 1982;18:377–380 PubMed
45. Ausman JI, Malik GM, Dujovny M, Mann R. Three-quarter prone approach to the pineal-tentorial region. Surg Neurol 1988;29:298–306 PubMed
46. Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery 1998;42:219– 224, discussion 224–225 PubMed
47. Dorward NL, Alberti O, Zhao J, et al. Interactive image-guided neuroendoscopy: development and early clinical experience. Minim Invasive Neurosurg 1998;41:31–34 PubMed
48. Matula C, Tschabitscher M, Day JD, Reinprecht A, Koos WT. Endoscopically assisted microneurosurgery. Acta Neurochir (Wien) 1995;134:190– 195 PubMed
49. Fukui M, Natori Y, Matsushima T, Nishio S, Ikezaki K. Operative approaches to the pineal region tumors. Childs Nerv Syst 1998;14:49–52 PubMed
50. Fukui M, Matsushima T, Fujii K, Nishio S, Takeshita I, Tashima T. Pineal and third ventricle tumours in the CT and MR eras. Acta Neurochir Suppl (Wien) 1991;53:127–136 PubMed
51. Yamamoto I, Kageyama N. Microsurgical anatomy of the pineal region. J Neurosurg 1980;53:205–221 PubMed
52. Jamieson KG. Excision of pineal tumors. J Neurosurg 1971;35:550–553 PubMed
53. Lapras C, Deruty R, Bret P. Tumors of the lateral ventricles. Adv Tech Stand Neurosurg 1984;11:103–167 PubMed
54. Neuwelt EA, Batjer HH. Pre- and post-operative management of pineal region tumors and the occipital transtentorial approach. In: Neuwelt EA, ed. Diagnosis and Treatment of Pineal Region Tumors. Baltimore: Williams & Wilkins, 1984:208–212
55. Reid WS, Clark WK. Comparison of the infratentorial and transtentorial approaches to the pineal region. Neurosurgery 1978;3:1–8 PubMed
56. Luo SQ, Li DZ, Zhang MZ, Wang ZC. Occipital transtentorial approach for removal of pineal region tumors: report of 64 consecutive cases. Surg Neurol 1989;32:36–39 PubMed
57. Stone JL, Cybulski GR, Crowell RM, Moody RA. The lateral position—dependant occipital approach—to pineal and medial occipitoparietal lesions. Technical note. Acta Neurochir (Wien) 1990;102:133–136 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








