Chapter 166 Posterior Lumbar Fusion by Open Technique
Indications and Techniques
The ultimate goal of a fusion is the elimination of pathologic segmental motion and its accompanying symptoms, achieved by the formation of osseous bridging across the previously mobile level.1 Successful fusion is known as arthrodesis; nonunion is referred to as pseudarthrosis. Surgical techniques have evolved dramatically over the past fifty years. However, despite these advances, there are three basic requirements for a successful fusion: immobilization, fusion bed, and bone graft.2 In the past, immobilization was accomplished with extensive bracing and casts; bracing is still used by some, but immobilization is often achieved by adjunctive instrumentation, mostly transpedicular instrumentation. Historically, the fusion bed and the bone graft were both accomplished through use of iliac crest bone graft; newer alternatives including (cadaveric) allograft bone, demineralized bone matrix, and bone morphogenetic protein (BMP) are now commonly used. This chapter focuses on the history of fusion, its indications, technique, perioperative management, and long-term sequelae.
History of Fusion
The history of modern spinal surgery, particularly fusion, began in the early twentieth century. The first fusion was described in 1911 by two independent surgeons, both of whom were treating patients with spinal tuberculosis, using different sources of bone graft: Albee used a tibial graft and Hibbs an iliac-crest graft (Table 166-1).3–4 The goal of the fusion was to provide stability, which was intended to limit deformity and the spread of tuberculosis. To obtain a successful fusion, these operations required a period of prolonged postoperative bed rest, as well as further immobilization by casts and braces.5 The first description of posterolateral lumbar fusion, involving fusion across the transverse processes, was in 1953 by Watkins.6
Table 166-1 Landmarks in the History of Fusion
| Date | Surgeon | Innovation |
|---|---|---|
| 1911 | Albee | First use of tibial graft |
| 1911 | Hibbs | First use of Iliac crest graft |
| 1953 | Watkins | First posterolateral fusion (bilateral transverse process fusion) |
| 1950s | Harrington | Development of instrumentation (used to treat pediatric scoliosis from polio) |
| 2002 | FDA approval of recombinant human bone morphogenetic protein-2 |
Harrington is credited with being the first to popularize spinal instrumentation: During the 1950s, he treated many children with scoliosis, primarily due to polio, using a complex instrumentation system composed of rods secured to the spine at two ends with sublaminar hooks, which became known as Harrington rods.7 Many variations on these instrumentation systems were then developed. Hartshill rectangles involved stainless steel rectangles held in place by sublaminar wires, which provided the structural integrity of the system.8 Subsequently, plates and then later rods were used in conjunction with transpedicular screws; additionally, translaminar or facet screws have also been used to promote a fusion. The approval of human recombinant BMP-2 (rhBMP-2) in 2002 by the Food and Drug Administration (FDA) is the most recent advance in fusion (see Table 166-1).
Indications for a Fusion
In this chapter, we discuss each of these pathologic degenerative conditions briefly, evaluating some of the key recent, high-quality outcome studies published (Table 166-2). An extensive discussion of the presentation and natural history of these conditions is out of the scope of this chapter; likewise, rather than an exhaustive list of all of the studies that have been performed regarding degenerative conditions, we have highlighted key trials. We limit our discussion of indications to degenerative and infectious conditions. The reader is to keep in mind that fusions are also used for treatment of patients with deformity, spinal trauma, and oncologic conditions, which are discussed in other chapters.
Spondylolisthesis
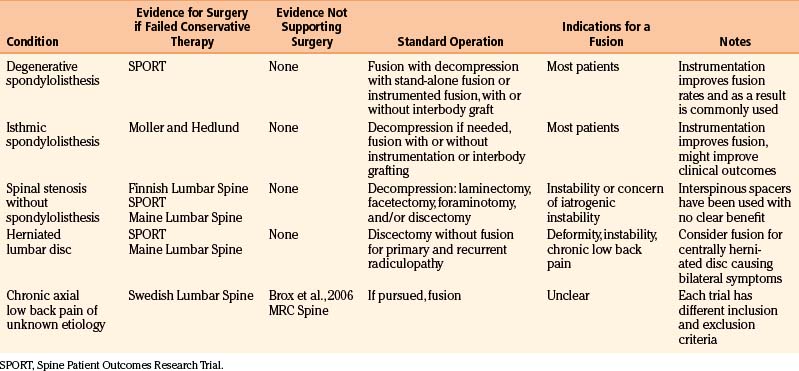
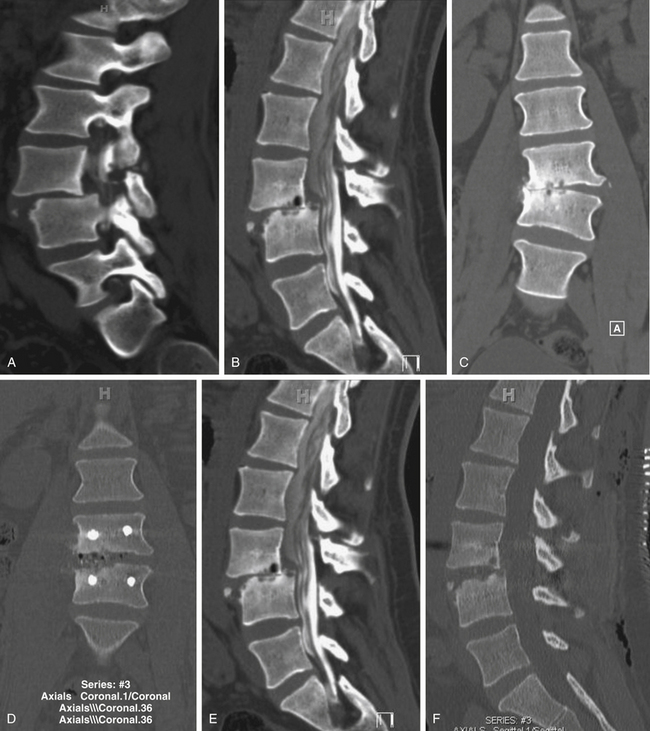
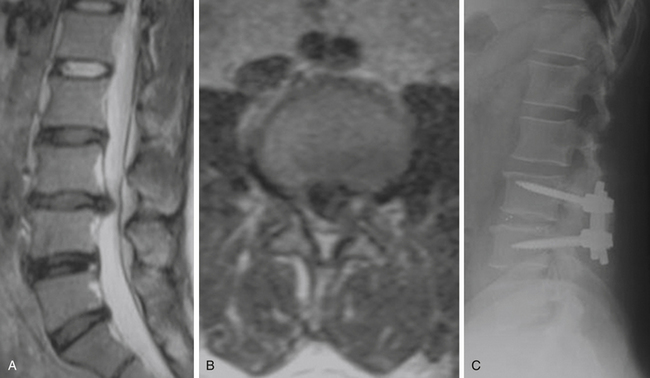
Spondylolisthesis is the anterior slippage of one vertebral body relative to the adjacent one. It can be divided into five different types based on etiology, first described by Newman and Stone: congenital, spondylolytic, traumatic, degenerative, and pathologic.9 The degree of spondylolisthesis is defined as the percentage of slippage of the vertebral body relative to the adjacent one, with grade 1 indicating only a 0% to 25% slip (Fig. 166-1), grade 2 a 26% to 50% slip, grade 3 a 51% to 75% slip, grade 4 a 76% to 100% slip, and grade 5 greater than 100% slippage (also referred to as spondyloptosis).10 Grade 1 or 2 spondylolisthesis is low grade, and grade 3 or higher is high-grade (Table 166-3). Although displacement can occur posteriorly or laterally, spondylolisthesis is synonymous with anterior displacement of the vertebral body.11
Table 166-3 Classification of Spondylolisthesis
| Grade | Percent Slippage | Classification |
|---|---|---|
| Grade 1 | 0%-25% | Low grade |
| Grade 2 | 26%-50% | Low grade |
| Grade 3 | 51%-75% | High grade |
| Grade 4 | 76%-100% | High grade |
| Grade 5 | >100% | Spondyloptosis |
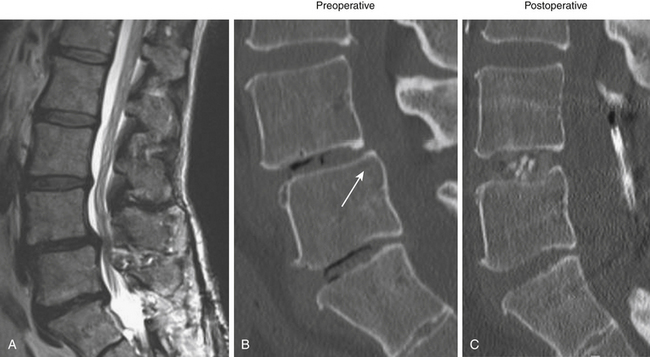
Degenerative low-grade spondylolisthesis is due to a combination of arthritic and degenerative changes in the disc and facet joints that leads to spinal stenosis and vertebral body displacement (Fig. 166-2).12 Degenerative spondylolisthesis is most often seen in patients older than 50 years and has an especially high incidence in women; the most commonly affected level is L4–L5 (the most mobile segmental level in the lumbar spine), and slippage is most commonly less than 30%. Patients typically present with neurogenic claudication, radiculopathy, low back pain, and/or vesicorectal dysfunction. Although listhesis can often be seen on lateral radiographs, further evaluation with computed tomography (CT) scans or magnetic resonance imaging (MRI) (or both) is often useful to exclude other concomitant sources of pain, such as herniated discs.11,13
The initial management of most patients with degenerative spondylolisthesis is nonsurgical. Conservative therapy should include a combination of physical therapy, nonsteroidal antiinflammatory drugs, and lifestyle modification, with most patients showing considerable pain relief. Surgical indications include persistent or recurrent back and leg pain with neurogenic claudication, substantially affecting one’s quality of life, and neurologic deficits, including bowel or bladder dysfunction. The goals of the operation are neural decompression to alleviate symptoms from the stenosis and stabilization to prevent further slippage and instability.11,13
The optimal treatment for patients with degenerative spondylolisthesis has been the subject of many recent studies (including prospective randomized, controlled trials), which provide some of the best evidence for lumbar spinal fusion: In appropriate candidates, surgical intervention is superior to nonoperative treatment.14–18 The highest quality data is provided by the Spine Patient Outcomes Research Trial (SPORT), comparing the effectiveness of surgical versus nonoperative treatment for patients with degenerative spondylolisthesis. Patients were selected from 13 centers in 11 states and were included if they had persistent symptoms (neurogenic claudication or radiculopathy) for greater than 12 weeks, as well as evidence of degenerative spondylolisthesis on lateral static radiographs. Enrollment was in either a randomized cohort or an observational cohort; treatment was decompression (with or without fusion) or standard nonoperative care. There was substantial crossover from patients who were randomized (as 66% of those randomized to receive surgery and 54% of those randomized to conservative treatment had an operation within 4 years), limiting the intention-to-treat analysis. However, the as-treated analysis, involving both the observational and randomized cohorts, showed a substantial and significant benefit to surgery in pain control, physical function, outcome measures [the Oswestry Disability Index (ODI)], patient satisfaction, and overall progress. These improvements were sustained 4 years after surgery.16–17 Although the significant crossover of patients negated the benefits of randomization, the outcomes reported from the SPORT trial likely represent the true clinical effectiveness of spinal fusion for patients with degenerative spondylolisthesis in the American population. The benefit of surgery over conservative treatment has been reported by other authors,18 advocated by experts,15 and included in guidelines for spine surgeons.14
The utility of spinal fusion to improve clinical outcomes in patients with degenerative spondylolisthesis has also been thoroughly investigated, showing that fusion is preferred.14 Although some authors have reported good outcomes in patients treated with decompression alone,19 laminectomy is likely to further destabilize an already unstable spinal segment, with potential worsening of the clinical and radiographic picture.20 Patients who undergo decompression alone have a significantly higher rate of reoperation than those who undergo decompression and fusion.21 A benefit to fusion has, in fact, been seen in all studies evaluating degenerative spondylolisthesis to date,12 with one exception—a retrospective trial comparing laminoplasty (rather than laminectomy) to posterolateral spinal fusion with instrumentation.18
In a landmark study comparing the outcome of decompression and fusion over decompression alone in patients with degenerative lumbar spondylolisthesis, Herkowitz and colleagues showed significant benefit in favor of fusion.22 Another prospective study found a significant improvement in the outcome measures (ODI and SF-36) in patients who had decompression with a posterolateral fusion compared to those receiving decompression alone.23 Several other cohorts of patients with degenerative spondylolisthesis treated with fusion have reported excellent outcomes,24–28 with substantial improvements in outcome measures as well as quality of life25,27; these benefits have persisted at least 5 years after surgery.28 A meta-analysis showed that fusion is significantly more likely to produce a satisfactory clinical outcome than decompression alone (relative risk, 1.40),12 and published guidelines recommend surgical decompression with fusion for appropriate candidates.14,20
Although a fusion is widely accepted for symptomatic patients, the need for instrumentation in achieving posterolateral fusion for degenerative spondylolisthesis has been debated. In the first major study evaluating the benefit of instrumentation in patients with this condition, Fischgrund and colleagues randomized subjects to posterolateral fusion with or without supplemental instrumentation. The use of instrumentation significantly improved fusion rates (82% in the instrumented versus 45%), but no differences were seen in clinical outcome.29 Another prospective randomized trial compared outcomes in patients with spondylolisthesis—both degenerative and spondylolytic—undergoing spinal fusionwith or without transpedicular instrumentation. Although a 5-year follow-up did not find any significant differences, patients with degenerative spondylolisthesis did significantly better with additional instrumentation. Patients with isthmic spondylolisthesis, however, did significantly worse with additional instrumentation. Reoperation rates were significantly higher in those treated with instrumentation, owing to misplacement or failure.30
Transpedicular instrumentation improved outcomes in patients who had a preoperative intervertebral angle difference of more than 11 degrees between flexion and extension in another study, but this benefit was not seen in patients with less motion.31 In yet another study, superior improvement in activities of daily living were seen in patients with transpedicular instrumentation, but there was no significant difference in overall outcome in those with transpedicular instrumentation.32 A meta-analysis confirmed an increased rate of solid fusion in patients with adjunctive instrumentation (relative risk 1.37), but it did not find an improvement in function.12 Interestingly, no difference in successful fusion rate between patients with unilateral versus bilateral one- or two-level fusions was reported in 2007, leading the authors to suggest that unilateral fusion decreases operative time and reduces complications.33
Kornblum and colleagues assessed the outcomes of patients who received a fusion without instrumentation who were treated in the trials of Herkowitz and colleagues and Fischgrund and colleagues. Average follow-up was almost 8 years. Interestingly, patients who had radiographic pseudarthrosis were significantly less likely to have a clinical outcome rated as excellent or good and performed significantly worse in symptom severity and physical function compared to those who had achieved successful radiographic arthrodesis.34
Many surgeons have used this study to advocate for the use of adjunct instrumentation in posterolateral fusion.15,35 The use of supplemental transpedicular instrumentation leads to higher rates of radiographic arthrodesis, a finding that has been associated with better clinical outcomes. Although there is only limited evidence that instrumentation directly improves outcomes in patients with degenerative spondylolisthesis,12 a survey of surgeon members of the North American Spine Society showed that the majority would recommend instrumented fusion,36 and the most recent guidelines suggest the addition of instrumentation to improve fusion rates.14,20,37
Interbody fusion has also shown good clinical outcomes in patients with degenerative spondylolisthesis, with successful union rates as high as 98%.38 Theoretically, the addition of an interbody graft increases the fusion surface area, potentially improving fusion rates.13 Studies that have directly compared posterolateral fusion with or without additional interbody fusion have not shown a clear benefit to one approach in comparison to the other. In one study, patients with preoperative segmental instability had significantly better outcomes with posterior lumbar interbody fusion (PLIF);39 another small study of 24 patients found a significant benefit to lumbar interbody fusion over posterolateral fusion alone.27 However, high rates of complications from interbody fusion for degenerative spondylolisthesis have also been reported. In one study, 8% of patients treated with interbody fusion had permanent neurologic deficits,40 leading the authors to conclude that complete facet takedown might limit morbidity associated with interbody graft placement. No clear data exist favoring the addition of interbody fusion to posterolateral fusion alone, and thus the decision is left up to the surgeon’s expertise and technical preference.
The use of an interspinous process distracter (whereby a spacer is inserted between the spinous processes for the treatment of spinal stenosis)41–42 in combination with nerve root decompression has been attempted. Although this approach has been shown to be superior to conservative management,43 high rates of further progression and surgical reintervention have been shown,44–45 indicating that this approach is likely inferior to fusion in patients with spinal stenosis and degenerative spondylolisthesis.
The treatment of adult patients with high-grade spondylolisthesis is challenging. Operative intervention should be considered in patients with severe pain, neurologic deficits, or progressive deformity.46 The degree of listhesis can cause technical difficulties in the operating room, and postoperative complication rates may be high. Posterior reduction and fusion without decompression has been reported with a low rate of pseudarthrosis (11.4%);47 others have used adjunctive fixation with iliac screws and/or transvertebral screws.48 The most commonly performed operation includes posterior instrumented fixation and fusion, an attempt at partial deformity reduction, and interbody structural support.46
Spondylolysis and Isthmic Spondylolisthesis
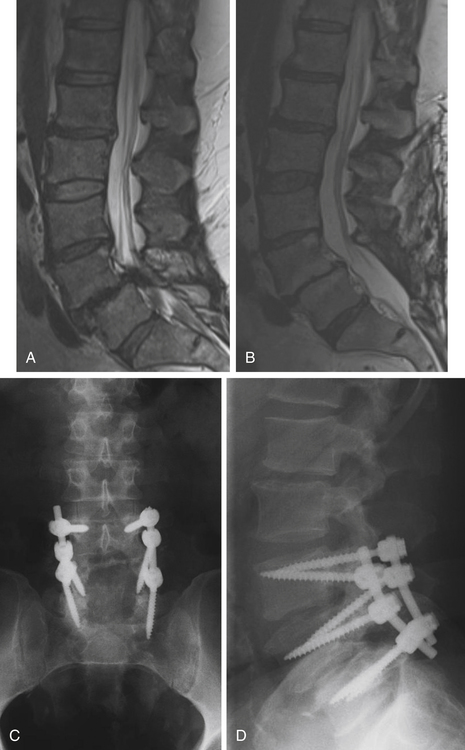
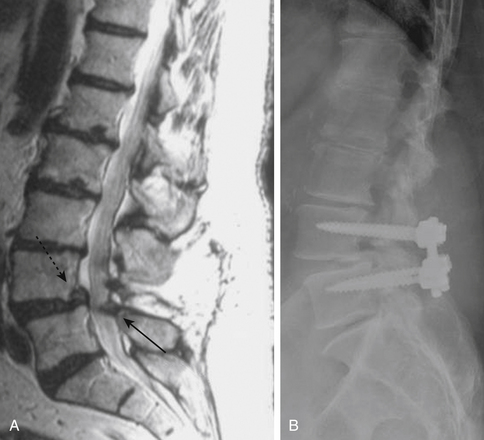
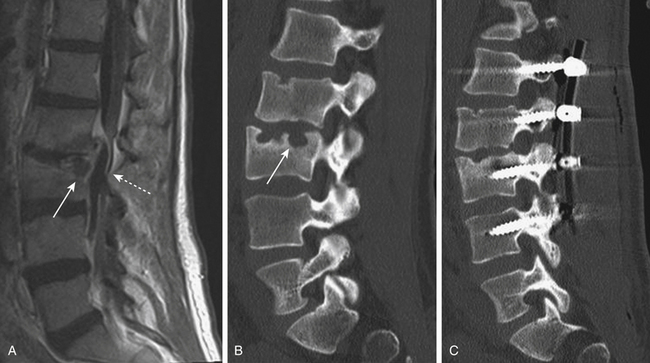
Isthmic spondylolisthesis is also common. The pars interarticularis, also referred to as the isthmus, is a thin bone of the posterior arch of the lumbar vertebrae where the lamina and the inferior articular process join the pedicle and superior articular process. A fracture in the pars interarticularis is referred to as spondylolysis, and can be found in 5% to 6% of the population. Isthmic spondylolisthesis results from elongation or traumatic fractures of the pars interarticularis, which lead to dissociation of the anterior and posterior vertebral arches. Anterior shear forces at the level of the listhesis eventually cause degeneration of the disc at the slipped level (Figs. 166-3 and 166-4). Patients are typically much younger than those in whom degenerative spondylolisthesis is seen; although there are a number of pediatric cases of isthmic spondylolisthesis, symptoms most commonly occur between the third and fifth decades of life. The same classification system for the degree of slippage is used in isthmic and degenerative spondylolisthesis, and whereas oblique radiographs can assess spondylolysis, CT is preferred. MRI is helpful for better views of the intervertebral discs and associated stenosis—centrally and foraminally.49–50
Similar to degenerative spondylolisthesis, patients with isthmic spondylolisthesis should have an initial trial of conservative management; precise indications for surgery have not been defined, but it is generally accepted that patients who have failed nonsurgical treatment for longer than 3 months, who have neurologic deficits, or who have significant disability are candidates for surgery.49
There is good evidence that patients with isthmic spondylolisthesis have better outcomes with surgery than with conservative management.51 Moller and Hedlund published a prospective randomized trial comparing patients (aged 18 to 55 years) with lumbar isthmic spondylolisthesis (of any grade), persistent symptoms for at least 1 year, and a “restricted functional ability.” These patients were randomized to posterolateral fusion or to an intensive exercise program; those randomized to posterolateral fusion were additionally randomized to include adjunct transpedicular instrumentation versus autologous iliac crest bone graft alone. At both 1 year and 2 years, those treated surgically had significantly improved pain scores and disability indices compared to those in the exercise group.51 In a long-term follow-up of the same patients (followed for 9 years after surgery), surgical patients had significantly better global assessment and self-satisfaction.52
Fusion is the preferred operation for patients with isthmic spondylolisthesis. Decompression alone is rarely used because it has been associated with poorer outcomes, up to a 27% slippage progression rate, and accelerated disc degeneration.49 Many other studies have reported excellent results in patients with isthmic spondylolisthesis who underwent a fusion.53–55 In one study, 75% of patients reported improvement after fusion.53 Another review of 132 patients followed for an average of almost 10 years found that improvements were seen in back and leg pain in 91.7% and 87.1% of patients, respectively; pseudarthrosis was only seen in 5%.54 Another study with an even longer follow-up (average 20.9 years) found 83% arthrodesis after posterolateral fusion, and only 7% of patients had moderate or severe disability.55 Patients who are working before surgery have been shown to have the best outcomes with fusion operations for isthmic spondylolisthesis; female patients and those who have not had an adequate trial of exercise might also have slightly poorer outcomes.53
The role of adjunct instrumentation to improve fusion in patients with isthmic spondylolisthesis remains debated. In Moller and Hedlund’s study, operative patients were further randomized to undergo stand-alone fusion versus transpedicular instrumented fusion; with a 2-year follow-up, there was no statistically significant difference in fusion rates or outcome in the two groups,56 and in the long-term follow-up of the same cohort, there was still no advantage to instrumentation.52 However, a 2005 meta-analysis showed a significant benefit to instrumentation: Fusion rates were higher (90% versus 77%), as was clinical success (85% versus 64%).57 Instrumentation can also aid in listhesis reduction, when attempted, which has also been argued to improve clinical outcomes in patients with isthmic spondylolisthesis.50 Thus, the use of supplemental instrumentation is preferred in patients with isthmic spondylolisthesis.
The addition of interbody fusion to posterolateral fusion is also debated. Good outcomes, including higher fusion rates, have been reported with PLIF.58 One prospective (but not randomized) study compared posterolateral fusion to PLIF, finding no difference in the two groups.59 Another retrospective study found no difference in clinical outcomes or fusion rate between PLIF and posterolateral fusion, although the PLIF group had better radiographic correction of subluxation.60 Superior clinical outcomes were seen in those treated with posterolateral fusion compared to PLIF in one retrospective study, although there was an insignificant trend toward higher fusion rates in the group treated with PLIF.61 On the other hand, a different prospective trial found better clinical outcomes at 6 months and 1 year after surgery in those treated with a combination of anterior lumbar interbody fusion (ALIF) and posterior instrumentation (although the benefit was not present at 2 years) compared to those treated with posterolateral fusion alone.62 Reviews have disagreed on the potential benefit of circumferential fusion,57,63 and thus, the addition of an interbody fusion is left to the surgeon’s experience.
Spinal Stenosis without Spondylolisthesis
Spinal stenosis without accompanying degenerative spondylolisthesis is distinct from degenerative spondylolisthesis with spinal stenosis. Spinal stenosis describes a narrowed spinal canal with neural compression. Typically occurring in middle-aged and older adults (although spinal stenosis can also be congenital), patients describe back and leg pain, numbness, occasionally weakness, and neurogenic claudication. Peripheral vascular disease, peripheral neuropathy, and degenerative conditions of the hip should be excluded; symptomatic patients should be evaluated by MRI, with or without additional CT scans.64–65
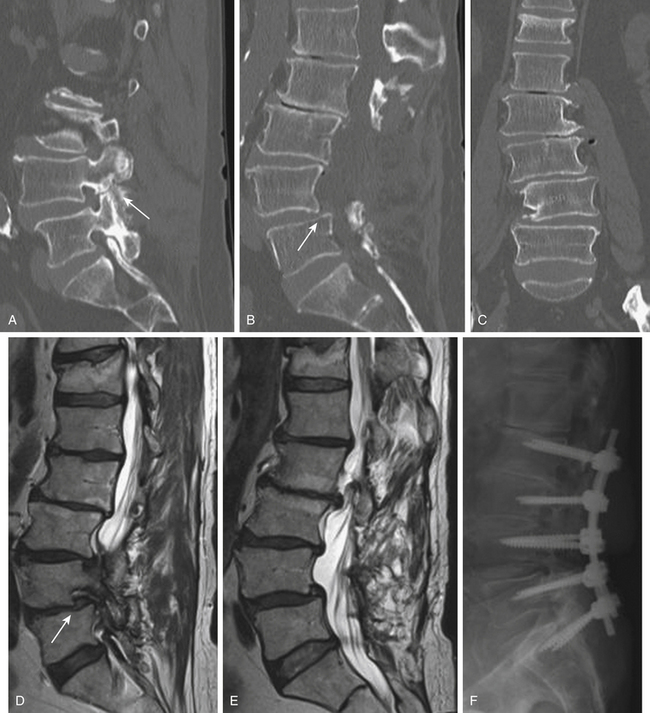
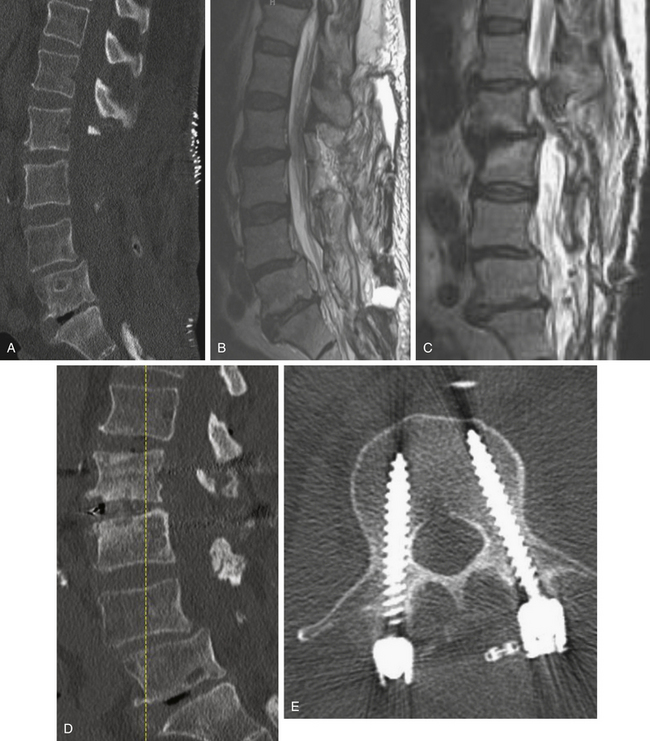
As with other degenerative conditions, the initial management should be nonsurgical. The presence of bowel or bladder dysfunction or neurologic deficits should merit prompt decompression; otherwise, surgery is reserved for those who have intractable pain or severely reduced quality of life and who have failed conservative therapy.65–66 The goals of surgery include pain relief, improved mobility, and prevention of neurologic deterioration. This is often accomplished by decompressive procedures, including laminectomies or laminotomies, partial facetectomies, foraminotomies, with or without discectomies. A fusion is added if there is concern for spinal instability or likely iatrogenic instability from the decompressive measures (Figs. 166-5 and 166-6).66–67
Two prospective randomized, controlled trials have evaluated the benefit to surgery in patients with spinal stenosis but without accompanying degenerative spondylolisthesis. The Finnish Lumbar Spine Research Group described 94 patients who were randomized to nonoperative treatment versus laminectomy of the stenotic segments, with or without instrumented fusion. At both 1 year and 2 years later, patients treated with surgery had greater improvement in leg pain, back pain, and overall disability; owing to the small size of the study, not all of the outcomes were statistically significant, but improvement in disability and back pain (but interestingly, not leg pain) were significantly improved in those treated with surgery.68
The SPORT group also included a trial of patients with lumbar spinal stenosis in the absence of spondylolisthesis who were randomized to decompression surgery without fusion or standard nonoperative care. In this trial, 289 patients were enrolled from 13 centers across the United States. The trial also had significant crossover between the randomized cohorts: the as-treated analysis, which negated the randomization but may be more indicative of outcomes in the American surgical candidates, showed a benefit to surgery in all primary outcomes that was sustained at 2 years.69 This group, moreover, found that surgical intervention was cost-effective compared to many other accepted surgical interventions, such as hip replacement.70
The Maine Lumbar Spine Study was another prospective cohort study comparing surgical treatment to conservative management in 148 patients. Those treated surgically had significantly better back or leg pain and overall satisfaction when followed up to 4 years, although the improvement seen with surgery was greatest initially.71 An 8- to 10-year follow-up of patients in the Maine Lumbar Spine Study found improvement in leg pain and greater back-related functional status seen in those treated surgically; however, by this time, improvements in low back pain and patient satisfaction were similar in those with and without surgical intervention.72 Predictors of good outcome after decompression may include greater walking capacity, milder symptoms, greater satisfaction with general health, and low cardiovascular risk profiles.73
The benefits of the addition of a fusion to a decompressive surgery in patients with spinal stenosis have been debated. Patients with preoperative instability or deformity likely benefit from the addition of a fusion. Extensive decompression can also cause iatrogenic instability, theoretically necessitating fusion (Figs. 166-5 and 166-7); however, supplemental fusion can increase operative times, blood loss, and complication rates as well as costs. Excellent results have been reported after decompression both with74–75 and without69,76 fusion in the treatment of patients with spinal stenosis.
One prospective study evaluating the benefit of laminectomy with or without arthrodesis in the treatment of lumbar spinal stenosis found that those with a fusion had a significant improvement in back pain at 2 months compared to those treated with decompression alone; however, in all other outcome measures there was no benefit to the addition of a fusion.77 Another study with longer follow-up found no significant benefit to the addition of either noninstrumented or instrumented fusion to decompression.78 A recent retrospective study comparing instrumented fusion to noninstrumented fusion found a significant benefit to surgery but no difference between those treated with adjunct transpedicular instrumentation versus iliac crest bone graft alone.75 Thus, the most recent guidelines on the management of patients with spinal stenosis without spondylolisthesis recommend decompression without additional fusion in patients without evidence of instability.65
The role of the addition of an interspinous spacer in addition to decompression in the treatment for spinal stenosis without spondylolisthesis has yet to be fully defined. One study did not find any difference in outcome measures in those in whom a supplemental interspinous spacer was placed.79
Herniated Lumbar Disc
Surgery is the optimal treatment for patients with persistent lumbar radiculopathy due to disc hernation. Both the Maine Lumbar Spine Study and the SPORT trial, which evaluated the treatment of radiculopathy, have shown a benefit to surgical intervention, which persists with long-term follow-up.80–84 The standard operation in these two trials was conventional open discectomy, which is discussed more extensively in the chapter covering lumbar discectomy.
The role of a fusion operation in patients with disc herniation is debated. Most commonly, patients are treated with discectomy alone, and good outcomes with a long period of follow-up have been described.85 However, fusion has been used as a treatment for patients both with primary and recurrent disc herniation. Disabling back pain can occur in up to 15% to 30% of patients who undergo a discectomy; 10% to 20% of patients who have had a discectomy have a subsequent lumbar spine operation.86 The intervertebral disc is the primary stabilizer of the functional spinal unit, decreasing biomechanical forces that are transmitted to the vertebral endplates. Thus, injury to the disc can theoretically lead to segmental spinal instability, and it may be the cause for chronic low back pain in these patients. Thus, some have advocated that a fusion also be performed in conjunction with discectomy for the primary treatment of patients with radiculopathy due to a herniated disc, although this is rarely performed today and is not our practice.87
Prospective trials that have compared discectomy to discectomy and posterolateral fusion in patients with radiculopathy (both primary and recurrent) have found no benefit to routine fusion in patients without instability, deformity, or chronic low back pain. One study evaluating 95 patients who had discectomy either with or without additional fusion found no benefit to fusion, with increased intraoperative blood loss, operative time, length of hospital stay, and total cost of the procedure for those who underwent a fusion.88 Excellent outcomes have also been reported for patients with recurrent disc herniation treated with discectomy alone,89 and one study reporting long-term (average follow-up of 89 months) outcomes in patients treated either by repeated discectomy or posterolateral fusion found no benefits to the addition of a fusion.90
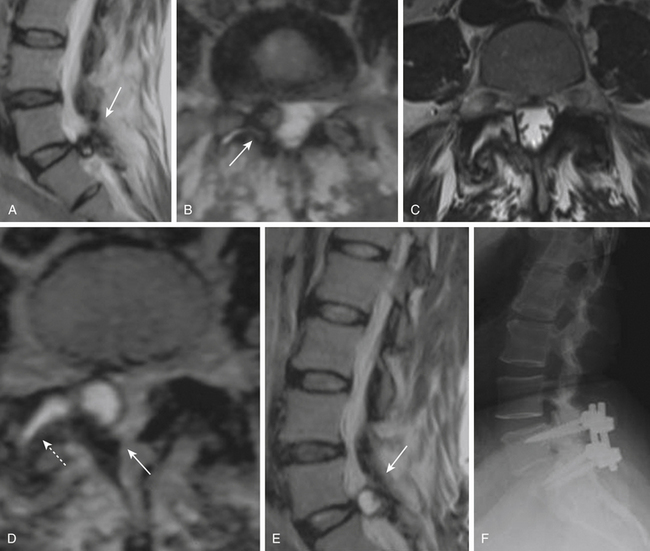
However, fusion might have a role in patients who have post-discectomy chronic low back pain. One group reported that patients with post-discectomy back pain had a 32% relief of pain and a 20% improvement in disability when treated with posterolateral fusion.86 In patients who present with recurrent disc herniation and chronic low back pain, it is reasonable to perform both a discectomy and a fusion of the spinal segment; guidelines also state that fusion should be considered in patients presenting with primary radiculopathy in whom there is evidence of deformity or instability.87 Patients who have a large, centrally herniated disc, causing bilateral radiculopathy or bowel and bladder dysfunction might also require a fusion (Fig. 166-8).
Chronic Low Back Pain
Low back pain is extraordinarily common. The lifetime prevalence of low back pain has been reported to be between 70% and 85%; annually it may be as high as 45%.91 In the vast majority of patients with low back pain, their pain is alleviated within weeks. A much smaller minority of patients—but still an important segment of the population—have chronic debilitating pain, hindering employment and producing a very poor quality of life.
Low-back pain is a symptom, not a disease.92 Even degenerative changes of the lumbar spine is a very broad term, including defined pathologic entities as well as nonspecific changes. The data evaluating surgery, particularly fusion, for patients with chronic low back pain of an unknown etiology are reviewed here. The vast majority of these patients have no accompanying neurologic symptom or radiologic evidence of spondylolisthesis, spondylolysis, spinal canal stenosis, foraminal stenosis, or nerve root impingement by a herniated intervertebral disc. Instead, they present with chronic, debilitating, and unremitting low back pain, and imaging, if notable, is only remarkable for nonspecific degenerative changes.
Management of such patients can be frustrating. Analgesics might only provide moderate pain relief, their side effects not withstanding. There is some evidence that patients with chronic low back pain improve with rehabilitation including cognitive and behavioral components. Data on acupuncture is conflicting but potentially promising. There is far less evidence in favor of a plethora of other interventions, including botulinum toxin injections, local injections, intraspinal steroid injections, epidural injections, facet joint or intradisc injections, radiofrequency denervation, or spinal cord stimulation.92–93
Surgery can alleviate chronic low back pain. The intervertebral disc may be a pain generator, and patients with chronic low back pain can have “discogenic” pain. A review of the contentious debate about discogenic pain is beyond the scope of this chapter. There are no clear indications on history, physical examination, or imaging as to which patients with chronic low back pain have a degenerative intervertebral disc as the source of their pain, because many people with severe degenerative changes of the intervertebral disc on imaging are asymptomatic. Preoperative discography has been used to assess benefit from a fusion, but Resnick and colleagues in 2005 guidelines did not recommend this practice.94
The benefit of a fusion surgery for patients with chronic nonradicular low back pain has been the subject of three randomized, controlled trials (Table 166-4). The evidence, however, raises more questions than it answers.
Table 166-4 Comparison of the Three Major Trials Investigating Patients with Chronic Axial Low Back Pain without Neurologic Symptoms of Unknown Etiology
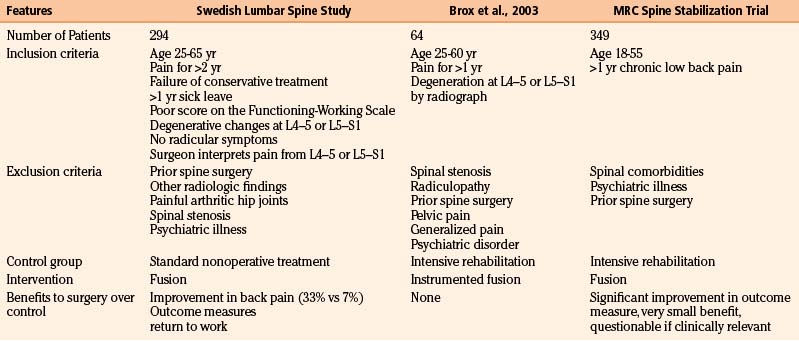
The first major randomized, controlled clinical trial evaluating the benefit of surgery in patients with chronic low back pain was by the Swedish Lumbar Spine Study Group, which enrolled 294 patients from 19 centers. Inclusion and exclusion criteria are listed in Table 166-4. Within the limited and generally very precisely defined population (although a surgeon’s interpretation of the anatomic location responsible for pain is quite vague), patients were randomized to nonoperative treatment—equivalent to being on a waiting list for surgery, with no precise intervention—versus one of three operative approaches. The results of the trial highly favored surgery. There was a statistically significant reduction in back pain in those treated operatively (although the clinical benefit was perhaps less impressive because only one third of patients improved, compared to 7% of the controls). Outcome measures and return-to-work rate also both significantly favored surgery.95 Not surprisingly, however, surgery was associated with significantly higher cost.91
The surgically treated patients in the Swedish Lumbar Spine Study were randomized to one of three operations: posterolateral fusion with or without instrumentation or posterolateral fusion with interbody fusion. No significant difference was seen among the three groups in outcome measures; patients treated with posterolateral fusion without instrumentation had significantly shorter operations, less requirement for blood transfusion, and shorter hospital stays than the patients treated with instrumentation or interbody fusion.96 With longer follow-up, patients treated with instrumentation or interbody fusion also had significantly higher rates of reoperation.97
Given the potential implications of this study, it is not surprising that it has been thoroughly evaluated.98–99 Although the trial did not compare surgery to any structured intervention,100 this might mimic clinical reality. The inclusion and exclusion criteria may be one of the main factors limiting how this study relates to the general population. Their definition of low back pain was, in fact, quite nonspecific;101 other criteria, however, were quite specific—such as the requirement that patients be out of work for at least 1 year—and might explain some of the differences seen compared with other trials.
Two other prospective randomized, controlled trials have investigated the benefit of surgery in the same population of patients, finding minimal or no benefit to surgery.102–103 The first trial was from Norway and enrolled 64 patients; inclusion and exclusion criteria are listed (and compared with the Swedish Lumbar Spine Study Group) in Table 166-4. Patients were randomized to either instrumented posterolateral lumbar fusion or an intensive rehabilitation program including cognitive behavioral interventions and exercise. Although there was a significant improvement in outcome measures postoperatively in the group treated with surgery compared with preoperative function, there was no difference between surgery and the rehabilitation group at 1-year follow-up.102
The MRC spine stabilization trial compared 349 patients who were randomized to fusion or an intensive rehabilitation program. The minimal inclusion criteria they used were age between 18 and 55 years and at least 1 year of chronic low back pain; exclusion criteria included spinal comorbidities, psychiatric disease, or previous surgical stabilization. There was a significant improvement in the primary functional outcome (ODI) in the patients treated with surgery compared to the rehabilitation group, but the improvement was of questionable clinical significance.103 Subgroup analysis found no difference in outcomes among those randomized to surgery who were treated with posterolateral fusion, interbody fusion, or flexible stabilization; however, posterolateral fusion had the lowest complication rate and cost.92,104
A meta-analysis combining these trials found a significant benefit to surgery, measured by an improvement in ODI; however, owing to the 16% pooled complication rate, surgery was not recommended by these authors.105 Recent reviews and guidelines also give conflicting recommendations, some favoring surgery106 and others saying there is insufficient evidence.100,107–108
Clearly, the best treatment for patients with nonradicular low back pain with nonspecific degenerative changes found on imaging has yet to be precisely defined. There are many possible reasons the data have been so contradictory. First, it is likely that in the absence of a clear pathologic etiology, such as spondylolisthesis, patients grouped into the category of “low back pain” probably have different foci responsible for their symptoms. If surgery is considered, patient selection is key, as well as understanding that the best scenario is likely only a moderate improvement in pain and function. Another reason the data are so divergent may be the wide variability in inclusion and exclusion criteria (see Table 166-4): The MRC spine stabilization trial included most patients who had back pain of unknown etiology for longer than 1 year; the criteria for the Swedish Lumbar Spine Study Group trial were more stringent. Because any benefit to surgery is likely to be only partial, some of their criteria—including disability preventing patients from working for at least 1 year, failure of conservative management, and persistent pain for 2 years—may in fact be quite critical to appropriate selection.
Osteomyelitis and Discitis
There has been a resurgence of spinal infections, in part owing to the increase in immunocompromised patients and the higher prevalence of antibiotic-resistant bacteria (including multidrug-resistant tuberculosis). Infections of the spine can involve the vertebral bodies, the intervertebral discs, the spinal canal, and the surrounding soft tissue (Fig. 166-9); pyogenic infections include bacterial and mycobacterial infections, and the nonpyogenic category encompasses fungi, yeast, and parasites. The presentation of spinal infection is variable but can include fever, focal back pain, and neurologic deficits (ranging from weakness to paralysis); risk factors include diabetes, urinary tract infections, steroid use, malignancy, alcoholism, and other immunocompromised states. Staphylococcus aureus is the most common organism (60% of cases); other important pathogens include Gram-negative rods such as Escherichia coli and Pseudomonas species, as well as Mycobacterium tuberculosis (with spinal tuberculosis seen in approximately 1% of patients with active disease). Fungal infections include coccidiomycosis, blastomycosis, histoplasmosis, cryptococcosis, and sporotrichosis (Table 166-5).109–110
| Bacterial organisms | |
| Fungal organisms | |
| Medical management | |
| Surgical indications | |
| Standard operation |
Precise indications for operative intervention in patients with spinal infections have not been defined. Generally, medical management is initially preferred, except in cases presenting with neurologic deficits, significant kyphotic deformity, or pathologic fractures (Fig. 166-10). Abscesses may need to be drained surgically. Medical management includes antibiotics, with or without supplemental bracing to aid in immobilization. CT-guided biopsy may be necessary to direct antibiotic treatment. Intravenous antibiotic treatment for bacterial infections should be continued for at least 12 weeks; treatment for tuberculosis should include 12 months of antimicrobial therapy if rifampin and isoniazid are used, but longer treatment may be necessary with different drugs. Patients with prolonged refractory infections despite optimal medical management might also be candidates for surgical treatment. The goals of operative treatment include débridement of infected tissue, adequate blood flow for tissue healing, and maintenance or restoration of spinal stability.110
Owing to the importance of spinal stability in preventing neurologic deficits and kyphosis, fusion operations are preferred, especially in patients with tuberculous spondylitis. The best surgical approach is debatable. Owing to the frequent involvement of anterior structures in spinal infections, the intervertebral discs and the vertebral bodies, as well as concerns that posterior decompression might lead to further deformity, some advocate anterior approaches.110
However, concern for reabsorption of the anterior graft and progressive kyphosis has led some to advocate the addition of posterior instrumentation to anterior fusion (thus creating a circumferential fusion) in patients with spinal infection. One small retrospective study found a higher rate of graft dislodgment in patients treated with ALIF alone compared to patients treated with ALIF plus posterior instrumented fusion.111 Another retrospective study evaluating patients with spinal tuberculosis found an increase in kyphosis and graft slippage in patients treated with ALIF alone; these complications did not occur in patients treated with ALIF plus posterior instrumentation.112 This approach has been advocated by other groups as well.113–114
Anterior approaches, with or without supplemental posterior instrumentation, are commonly used for patients with spinal infections, but others have also reported the use of transpedicular decompression and posterior instrumentation for vertebral osteomyelitis.115–118 One group used this approach in patients who had spinal tuberculosis that manifested only with mild kyphosis or early bone destruction, reporting 100% fusion rates and substantial correction of kyphosis.115 Advantages of a single-stage posterior approach include easy access to the spinal canal for neural decompression, prevention of loss of corrected vertebral alignment, and facilitation of early mobilization.116
The use of instrumentation for spinal infections was initially controversial lest it serve as a nidus for persistent infection. However, instrumentation has been successfully used without additional complications in many cases,109 including both transpedicular instrumentation and interbody cages.119 Although infection is currently listed as a contraindication to rhBMP-2 use, it has been used with excellent fusion rates, good clinical outcomes, and no additional complications in patients with pyogenic vertebral osteomyelitis.120–121
Assessment Before a Fusion Operation
Preoperative medical clearance is essential. The most recently published data from national databases have shown that more than half of in-hospital perioperative complications are cardiac, pulmonary, or renal in nature, occurring in patients with chronic conditions.122 In addition to standard preoperative workup, nutritional status should be assessed prior to a fusion, because poor nutritional status places patients at higher risk for complications and pseudarthrosis. A serum albumin level less than 3.5 g/dl and a total lymphocyte count lower than 2000 cells/mm3 are indications that nutrition is suboptimal.123 Owing to the possibility of longer operative times and higher blood loss from instrumented fusion cases, some have advocated predonation of blood for allogeneic transfusion. A recent retrospective case-control study showed no benefit to this practice in patients with a normal coagulation profile.124
Smoking and Fusion
Many studies have shown a detrimental effect of smoking on the fusion rates and outcomes of patients undergoing lumbar fusion. The ability of cigarette smoke to hinder fusion has been seen in a number of animal models125 and may be related to alterations in gene expression or the antiinflammatory effects of smoking.126 Glassman and colleagues showed a significantly increased rate of pseudarthrosis in patients who continued to smoke after surgery (14.2% in nonsmokers versus 26.5% in smokers); lower return-to-work rates were also seen in that population. However, this difference was not present in patients who quit smoking for more than 6 months after their operation compared to nonsmokers.127 Another study showed poor fusion rates and lower patient satisfaction in smokers compared with nonsmokers after instrumented posterolateral fusion, with smoking cessation increasing fusion rates close to those seen in nonsmokers.128 Osteoinductive biological bone graft alternatives might partially overcome the inhibitory effects of smoking. A study evaluating the fusion rates using rhBMP-2 versus iliac crest bone graft in smokers and nonsmokers showed an increased rate of fusion in smokers treated with rhBMP-2 over iliac crest bone graft (95.2% versus 76.2% respectively), but smokers still had consistently poorer clinical outcomes, even if treated with rhBMP-2.129 Although these results are promising, smoking cessation is the best way to improve fusion rates.
Diabetes and Obesity
Diabetes and obesity are common comorbidities in America130 and are becoming increasingly prevalent throughout the world. Both have been shown to increase complications after fusion—especially instrumented spinal operations—and thus preoperative medical optimization of blood sugar and weight loss are crucial.
Type 1 diabetes mellitus (DM type I) and type 2 diabetes mellitus (DM type II) were both shown in a retrospective case-control series to have significantly increased complications after posterior fusion with instrumentation (56% in the T1DM patients versus 21% in controls). Rates of pseudarthrosis were also significantly higher in diabetics: 22% in T2DM subjects, 26% in T1DM subjects, and 5% in controls.131 Another study noted high rates of complications in diabetic patients undergoing instrumented posterolateral fusion (31%), but it also found 75% of patients rated their outcome as excellent or good and 95% of patients achieved radiographic fusion.132 Much of the increased risk of complications comes from an increased rate of surgical site infections (relative risk, 4.10).133 Other hospital complications reported from a large review of the nationwide inpatient sample (NIS) include pneumonia, need for transfusion, and nonroutine discharge from the hospital; diabetes was also significantly associated with higher total costs and longer length of hospital stay.134 Interestingly, subclinical elevations of hemoglobin A1c (above 6.1%) were also found to be significantly associated with increased cost and prolonged hospital stay in patients undergoing lumbar decompression and fusion, suggesting that routine screening of hemoglobin A1c may be beneficial.135
Obesity is also associated with perioperative complications. In a retrospective review of overweight and obese patients, complication rates were found to be directly correlated with body mass index (BMI), independent of hypertension or diabetes: 14% with a BMI of 25, 20% with a BMI of 30, and 36% with a BMI of 35. Some of the complications include pseudarthrosis, wound infection, cerebrospinal fluid leak, deep vein thromboses, cardiac events, pneumonia, urologic issues, and positioning-related nerve palsies.136 A study from the NIS database found higher BMI to be associated with increased transfusion requirements and increased likelihood of discharge to assisted living in patients undergoing fusion; morbidly obese patients also had an increased rate of wound complications and perioperative infections. Rates of thromboembolic events, a known complication of obese surgical patients, were not examined in that study. Perioperative mortality and length of stay were not different, however, suggesting that obese patients are safe surgical candidates.137 Surgical complications and length of stay may be decreased in obese patients undergoing minimally invasive lumbar spinal fusion surgery, suggesting this approach, when clinically indicated, may be considered in obese—especially morbidly obese—patients.138
End-Stage Renal Disease
The prevalence of patients with end-stage renal disease (ESRD) on hemodialysis is increasing, largely owing to the prevalence of hypertension and diabetes mellitus; the shortage of kidney donors places patients with ESRD on hemodialysis for extended periods. Both osteoarthritis and osteoporosis are common in patients with ESRD: osteoarthritis is seen in 23% of patients on hemodialysis; osteoporosis is due to secondary hyperparathyroidism, dynamic bone disease, and steroids. Han and colleagues showed that patients with ESRD have acceptable outcomes after spinal surgery that can improve quality of life; however, morbidity and mortality were higher and fusion rates were comparatively lower, pointing to the importance of preoperative clearance, medical management, and nephrology consultation.139
Owing to the high rates of hypertension, diabetes and cardiac disease, patients with ESRD need these comorbidities managed; echocardiography and possible coronary angiography should be considered. Medical management of osteoporosis should include maintaining calcium, vitamin D, and phosphate levels within normal (with a low-phosphorus diet and phosphate-binding agents if needed). Vitamin D and calcitriol should be used in patients with secondary hyperparathyroidism, and hormone replacement therapy should be considered; bisphosphonates should not be used in patients with ESRD owing to the drugs’ nephrotoxicity and the possibility of hypocalcemia. The goal hemoglobin preoperatively is 10.0 g/dl, and patients should receive appropriate transfusion or treatment with Epogen. Perioperative potassium level should be maintained below 5.0 mEq/L; patients should undergo routine dialysis between 18 and 24 hours before surgery (and sodium polystyrene [Kayexalate] should be administered as necessary) and then dialyzed again 18 to 24 hours after the operation. Postoperatively, ESRD patients should be admitted to the intensive care unit with frequent sodium and potassium checks, because up to half of patients have postoperative hyperkalemia despite these measures (Table 166-6). Other complications reported included spondylodiscitis, postoperative delirium, and an 8% mortality rate. Fusion rate was 57%.139
Table 166-6 Management of Patients Who Have End-Stage Renal Disease and are Undergoing Fusion
| Comorbidity | Management |
|---|---|
| Vascular or cardiac disease | |
| Osteoporosis | |
| Anemia | Transfuse to keep hemoglobin >10.0 |
| Hyperkalemia |
Rheumatoid Arthritis
Cervical spine disease is well known in patients with RA;140 the lumbar spine is also commonly affected, manifested as degenerative scoliosis, degenerative spondylolisthesis, disc space narrowing, facet erosion, compression fractures, or spinal stenosis. Many patients with RA are treated with corticosteroids, predisposing them to osteopenia and osteoporosis. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used analgesics in patients with inflammatory arthritis; however, because chronic NSAID use has been associated with poorer rates of successful fusion, discontinuation of these agents should be considered.141–144 Disease-modifying drugs such as methotrexate and newer regimens that include anti–tumor necrosis factor-α (TNF-α) drugs are potent immunosuppressants. Crawford and colleagues published a case-control series that retrospectively evaluated RA patients at a major spine center. Although clinical outcomes and fusion rates were similar in patients with and without RA, complications were higher (37% versus 21%), including wound infections and implant complications (16%).145 The most recent rheumatologic guidelines suggest discontinuing any anti-TNF-α agents 2 to 4 weeks before surgery,146 a more prolonged cessation may be necessary in spine surgery to avoid postoperative complications such as discitis.147
Fusing the Aging Spine
As the generation of children born after World War II (known in the United States as the “baby boomers”) age, the percentage of the population that is older is increasing and will continue to do so both in America and elsewhere. The incidence of lumbar spine disease in older adults is very high, especially degenerative spondylolisthesis with spinal stenosis, which often requires a fusion.148 The impact of lumbar spine disease on quality of life can be severe. The recent finding that patients who have had lumbar spine surgery have a lower mortality than the general public has led some to suggest that treating degenerative spine disease is important in keeping patients ambulatory.149
Treating the aging spine, however, has many special considerations. The risk of complications increases with each comorbidity, and it is not uncommon to have older adults with more than three major comorbidities. Additionally, the prevalence of osteoporosis (defined as a t-score less than −2.5 by dual energy x-ray absorptiometry [DEXA] scan) is very high in older adults.150 Owing to decreased bone strength seen in osteoporotic spines, some have advocated non-instrumented fusion. A recent study showing a lack of increased complications with rhBMP-2 in older adults might lead to its wider use in the geriatric population.151 However, many studies have also shown that augmenting transpedicular instrumentation with agents such as polymethyl methacrylate (PMMA) is even more beneficial in osteoporotic patients.152–153
Given the high prevalence of multiple chronic medical conditions in the elderly, it is important to assess the safety and the effectiveness of lumbar spine surgery, especially fusion. One retrospective review of perioperative complications showed a 35% rate of major complications in patients in their ninth decade of life undergoing a fusion, leading the authors to question the safety of fusion in this population.154 Other studies, however, have found much lower rates of complications. Another study that analyzed patients older than 65 years at a single hospital only found a 3% risk of major complications, the most serious of which was an epidural hematoma. Within the subpopulation of patients older than 80 years, there was no statistically significant increase in the rate of complications.155 There was no significant difference between the rate of complications seen in patients older and those younger than 65 years in another study; the only difference they saw was a longer length of stay in the geriatric patients.156 Lumbar fusion surgery is safe in the geriatric population and associated with an acceptable risk profile; however, older adults should also be screened carefully and their medical comorbidities optimized to increase the likelihood of a safe operation.
Excellent outcomes after spinal fusion have been reported in older patients. Sustained improvements in functional outcome measures, including ODI and SF-36, have been described in an exclusively geriatric cohort of patients.157 Similarly, in a study of primarily older adults, high successful fusion rates were reported (82%) in the setting of improvements in the SF-36 that persisted 2 years after surgery.158
Most promising, however, is a study published from a group in Korea. The group assessed the impact of lumbar spine surgery on mortality. Using the Kaplan-Meier method, they compared the 10-year morality of more than 1000 patients to age- and sex- matched controls. They found that in comparison to the controls, standardized mortality ratios all favored surgery: 0.21 for patients aged 50 to 59 years, 0.53 for patients aged 60 to 69 years, and 0.45 for patients aged 70 to 85 years. Additionally, patients who had a fusion had a statistically significant lower mortality than those who had decompression alone, although this might relate to surgeons being less likely to offer high surgical risk patients a fusion procedure. The authors compare the benefit of lumbar spine fusion to studies that have shown that patients who have hip replacement also have a reduced mortality, hypothesizing that a key factor may be the improvement in ambulation allowing patients to participate in regular exercise.149 The ability to generalize data from the South Korean population to American patients, however, may be limited, because rates of diabetes and obesity are lower in South Korea and life expectancy is longer.
Adjuncts to improve fusion have been used in the geriatric population. RhBMP-2 has been shown to decrease complications and, surprisingly, cost, in comparison to iliac crest bone graft in one prospective randomized clinical trial.151 As described in more detail later, direct-current electrical stimulation (DCS) has also been studied in older adults and found to improve clinical outcomes, namely median walking distance, although fusion rates were not improved.159–160
In addition to greater risk of complications, older adults also have a much higher rate of osteoporosis. Another study from Korea showed that in the population at the authors’ institution, among patients older than 50 years, 14.5% of men and 51.3% of women had osteoporosis confirmed by DEXA scan.150 Patients with osteoporosis need comprehensive medical management before surgery. At least 1200 mg of calcium and 1200 IU of vitamin D should be taken daily. Raloxifene, a selective estrogen receptor modulator, has been shown to reduce the risk of vertebral fractures and should be considered in patients who do not have an underlying disposition to or history of venous thromboembolism. Bisphosphonates have also been shown to decrease the rates of vertebral fractures; alendronate 70 mg once weekly and risendronate 35 mg weekly are the most commonly used regimens. Other options that are less commonly used include nasal insufflation of calcitonin and daily subcutaneous injections of parathyroid hormone, both of which are best prescribed in conjunction with endocrinologists.161 Medical management should continue in the postoperative period; although there has been some concern that bisphosphonates might inhibit fusion, animal models do not suggest this to be the case.162
The preoperative planning of fusion cases requires special consideration in patients with osteoporosis. Some surgeons are reluctant to use instrumentation in this population owing to the risks of instrumentation failure (Fig. 166-11). However, successful use of transpedicular instrumentation has been described in the geriatric and osteoporotic population, sometimes using less-rigid rods.163–164 Strategies to supplement transpedicular instrumentation have also been used. Some have described the use of transpedicular cement injection, creating a vertebroplasty-like effect by strengthening the vertebral body and providing additional stability to the anchoring of the screws.165 Similarly, PMMA has also been shown to augment cannulated pedicle screws with successful arthrodesis demonstrated radiologically; the major additional risk of this procedure is a theoretical pulmonary cement embolism.153,166 Balloon kyphoplasty augmented screws have also been proposed, but cadaveric studies suggest PMMA augmentation may be superior.152,167–168
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree