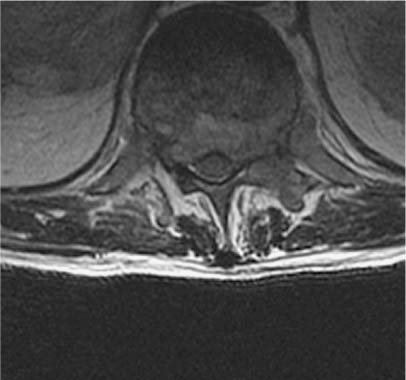
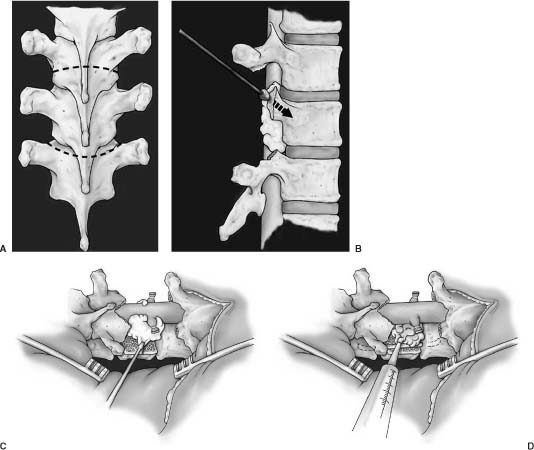
33 Surgery for metastatic spine tumors is generally undertaken as a palliative procedure to improve the patient’s quality of life, maintain or improve neurologic function, and relieve pain. The primary goals of operation are spinal cord decompression, immediate and durable spine stabilization, elimination of brace dependency, and obtaining local tumor control. Early attempts at spinal decompression using laminectomy alone or in combination with adjuvant radiation therapy (RT) proved to be no better than RT alone.1–18 Poor outcomes using laminectomy resulted from the inability to resect vertebral body and epidural tumor, resulting in persistent spinal cord compression. Further, resection of the posterior elements without instrumentation often leads to segmental instability, resulting in progressive kyphosis and increased neurologic deficits. Patients undergoing laminectomy for decompression of metastatic tumor had only a 60% chance of maintaining ambulation and a 33% rate of return to ambulation.2–18 In some series, over 50% of patient were made worse with operation. In addition, pain relief was rarely achieved due to persistent or iatrogenic instability. Advances in surgical techniques have improved upon laminectomy outcomes, primarily using posterolateral19–29 and anterior transcavitary19–22,30–41 approaches, by providing wider exposure for spinal cord decompression, resection of paraspinal masses, and spinal stabilization. Reconstruction following these aggressive approaches is achieved using rigid posterior segmental fixation and anterior instrumentation. Patients undergoing such approaches have a 95% chance of maintaining and a 60% chance of regaining normal neurologic function; less than 5% of cases are made worse. Furthermore, patients report significant pain improvement in greater than 75% of operated cases. The decision to use a posterolateral, anterior transcavitary, or combination approach is based on the location of bone, epidural, and paraspinal tumor, the type of reconstruction required, patient comorbidities, the extent of disease, and the surgeon’s familiarity with the techniques. The anterior transcavitary approaches provide excellent exposure for resection of lytic or sclerotic tumor in the vertebral body, as well as anterior paraspinal masses. Epidural tumor can be safely resected when it is located anterior or anterolateral to the dura on the side of the approach; however, it is difficult to resect some of the more common patterns of epidural tumor that extend bilaterally (270 degrees) or circumferentially around the cord. Additional technical issues include dissecting through the tumor to decompress the normal dura and difficulty with identifying and resecting tumor from functional nerve roots in the lumbar spine. Patients must also be able to tolerate an anterior approach from a pulmonary standpoint and have retroperitoneal structures (i.e., bowel, aorta) mobilized that may be scarred from prior RT or operation. Anterior approaches have been described for the thoracic and lumbar spine at every level, such as trapdoor (T1–T3) or thoracoabdominal approaches (T11-L1). These approaches may require the specialized services of thoracic or general surgeons. Anterior reconstruction often provides excellent fixation; however, for tumors involving the anterior and posterior spinal elements, circumferential fixation is indicated. The poor outcomes with laminectomy made many surgeons wary of using a posterior approach in cancer patients, but the posterolateral approach has great utility in treating these patients. In addition to resection of the spinous process and lamina(e) (i.e., laminectomy), the posterolateral approach extends the bone decompression to include the pedicles and facet joint. This approach provides a wide exposure to resect 270-degree or circumferential epidural tumor. Epidural tumor decompression can be achieved starting from a normal dural margin, and encased nerve roots can be readily identified and preserved in the lumbar spine and lumbosacral plexus. The posterolateral approach can be extended to include vertebral body resection [Posterolateral transpediculor approach (PTA) circumferential decempression and fixation] and/or chest wall resection. In our practice, the PTA approach has been used extensively for patients with vertebral body tumor who cannot tolerate an anterior approach because of concomitant chest disease, poor pulmonary function, unresectable paraspinal masses, or previous chest or retroperitoneal surgery. Patients with three-column and discontinuous sites of tumor involvement can be resected from a PTA approach. Circumferential instrumentation can be placed from this approach, which provides immediate and durable fixation. Further, the approach is the same for all segments of the thoracic and lumbar spine and does not require specialized assistance for exposure. Operative decision-making depends on information obtained from preoperative images. Magnetic resonance imaging (MRI) scans are the most sensitive and specific for detecting spinal tumors and are extremely useful for operative planning. Routine imaging for metastatic tumor includes a sagittal screen of the entire spine from C1 to the sacrum with axial cuts through suspicious areas. The three imaging sequences assessed prior to operation are T1-weighted, T2-weighted fast spin echo, and T2-weighted, fat-suppressed short tau inversion recovery (STIR) sequences. T1-weighted (hypointense) and STIR images (hyperintense) are the most useful for identifying sites of bone disease on sagittal images. T2-weighted axial images are the most useful for assessing the degree of epidural spinal cord compression. Tumor extending from the vertebral body to compress the spinal cord often leaves the posterior longitudinal ligament (PLL) intact. This can be visualized on T2-weighted images as a hypointense line between the anterior tumor and posterior thecal sac, with tumor extending laterally around the spinal cord. We refer to this as the “V” sign, and it indicates that the PLL must be opened to adequately resect the anterior compressive tumor (Fig. 33-1). We have not found contrast administration helpful, except for evaluating leptomeningeal tumor. Contrast administration on T1-weighted images may cause tumor signal to become isointense relative to normal marrow and obscure its presence. FIGURE 33-1 T2-weighted magnetic resonance imaging (MRI) shows the typical pattern of tumor extending from the vertebral body, pushing the posterior longitudinal ligament (PLL) posteriorly to compress the dura. This creates the “V” sign and signifies that tumor does not directly abut the anterior dura. A margin on the anterior dura can be obtained by resecting the PLL. Plain x-rays should be obtained to assess the sagittal and coronal alignment of the spine but is not sensitive enough to detect tumor in most cases. Dynamic flexion and extension films are not routinely used in symptomatic patients with spinal cord compression for fear of exacerbating neurologic deficits. Computed tomography (CT) scans are predominantly useful for differentiating sclerotic from lytic tumor. In our practice, myelography is used to assess for recurrent tumor following the placement of instrumentation or in patients with a contraindication to MRI (e.g., pacemaker). In patients with titanium hardware, one can often visualize the spinal canal and tumor recurrence on MRI without resorting to myelography. Angiography and embolization are used selectively based primarily on tumor histology. Radiographic criteria such as bright contrast enhancement, signal voids representing blood vessels, or evidence of hemorrhage within the tumor on MRI scans may also imply increased vascularity and indicate the need for embolization before surgery.42 However, in a series of 51 patients with hypervascular tumors embolized over a 5-year period at our institution, MRI findings were often not predictive of tumor vascularity. Tumor histology may be more important than MRI findings for using preoperative embolization. Tumors routinely embolized include renal, follicular or papillary thyroid, pheochromocytoma, paraganglioma, angiosarcoma, and leiomyosarcoma. Common tumors such as lung, breast, prostate, and colon are relatively avascular and do not routinely require embolization. Multiple myeloma and lymphoma are vascular tumors but have predominantly a capillary blood supply, and obliteration of the segmental feeding vessels does not significantly reduce bleeding in these tumors. Patients undergoing PTA following embolization seemed to have little tumor bleeding but increased venous bleeding, both epidural and in the muscle. Review of embolization films shows significant arteriovenous shunting in these patients, which probably accounts for this phenomenon. Preoperative medical clearance is important for good outcomes. Patients with metastatic tumors often have medical and oncologic comorbidities. Preoperative pulmonary function tests and cardiac assessment are obtained when indicated. Issues unique to the cancer population include laboratory abnormalities resulting from chemotherapy, radiation, and bone marrow infiltration. Neutropenia can often be treated with administration of Filgastrim (Amgen, Thousand Oaks, CA), which is a granulocyte colony-stimulating factor (G-CSF). This can often be administered 48 hours preoperatively to raise the absolute neutrophil above 1.0. Thrombocytopenia is also a common problem. Discontinuing common drugs such as heparin and Bactrim may improve platelet counts without the need for transfusion. Commonly platelet counts also improve spontaneously 2 to 3 weeks following chemotherapy. For emergency procedures, platelets are transfused to keep levels above 100,000. Patients are positioned prone on lateral chest rolls, and the head is affixed in the neutral position in the May-field fixation device. Placing the head in skull pins allows anesthesia access to the nasopharynx throughout the procedure and limits the pressure to the forehead and orbits that is seen with horseshoe immobilization. Warming blankets are placed over the lower extremities and all fluids are filtered through warmers to prevent hypothermia. Arterial pressure monitoring and central venous access are routinely used. All patients are given perioperative steroids and intravenous antibiotics. A midline incision is centered over the level(s) of tumor involvement and extended at least two levels cepha-lad and caudad to prepare for instrumentation. The ligamentous attachments and muscle are taken off the spinous processes and laminae to the tips of the transverse processes at all exposed levels. The mean arterial blood pressure is lowered to 70 mm Hg to reduce paraspinal muscle bleeding and then is allowed to return to normotension following muscle dissection. The rib heads are not exposed unless a chest wall resection is required. If the posterior elements are involved with tumor, care must be taken to dissect the ligamentous attachments and muscle off the tumor without transmitting pressure to the spinal cord. This is often done with a right-angle clamp and Bovie cautery. If present, posterior element and adjacent soft tissue tumor are then resected piecemeal to the level of the lamina. Prior to tumor decompression, the levels exposed for fixation should be packed with laparotomy pads to reduce bleeding during the decompression. The posterior bone work is initiated by removing the spinous processes with a rongeur. The high-speed drill is then used to remove the lamina(e) extending over the levels of tumor involvement or the levels of the adjacent disc spaces if a PTA is considered (Fig. 33-2A). Using a side-cutting bur with a blunt tip, such as the M-8 bur on the Midas Rex drill (Fort Worth, TX), the lamina can be drilled to a thin cortical shell or all of the bone removed to expose the ligamentum flavum, dura, or epidural tumor. The thin cortical shell can be removed with a 2-mm Kerrison rongeur, but the presence of an anterior mass compressing the spinal cord prohibits the use of large Kerrison rongeurs in the spinal canal. Bilateral pedicle resections are accomplished by coring the cancellous bone of the pedicle with the drill and then removing the cortical bone adjacent to the spinal dura or tumor. The superior and inferior facets are then removed, exposing the lateral dura (Fig. 33-2B). In the lumbar spine, a unilateral pedicle resection and facetectomy are often sufficient to gain exposure to epidural and vertebral body tumor. Following bone removal, the ligamentum flavum and epidural tumor are resected with tenotomy scissors starting at the interface between the tumor and uninvolved dura. Bipolar cautery on a low setting is occasionally useful to help define this interface. Tenotomy scissors have a sharp point that can be used to readily dissect tumor from the dura when used with a gentle spreading motion and then can be turned perpendicular to cut and resect tumor. Often the epidural venous plexus can be identified and coagulated in a bipolar fashion, preventing excessive bleeding.
Posterolateral Approaches to the Thoracic and Lumbar Spine
 Operative Technique and Procedure
Operative Technique and Procedure
Surgical Approaches
Preoperative Imaging
Preoperative Planning
Posterolateral: Patient Positioning and Exposure
Tumor Dissection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




