Chapter 29 Prenatal Diagnosis of Structural Brain Anomalies
Introduction
The fetal brain develops during the course of pregnancy, from its primitive three-vesicle stage into a complex structure with an array of sulci and gyri resembling the adult brain by the end of gestation [Nadich et al., 1994]. Fetal brain development is characterized by well-defined, genetically programmed, morphological and maturational changes, including neuronal migration, sulcation, and myelination that can be studied by ultrasound, starting from gestational week 6–7 until delivery [Ertl-Wagner et al., 2002]. Fetal magnetic resonance imaging (MRI) enables good demonstration of anatomy, particularly of cortical development and diagnosis of diverse anomalies, starting at around 18–20 weeks of pregnancy.
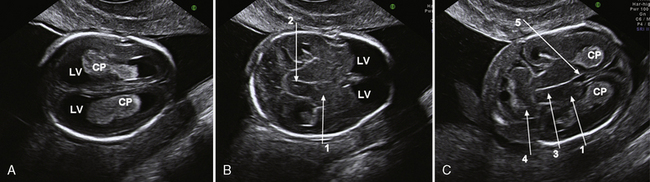
Sonography is the most important imaging method for prenatal malformation screening. It is noninvasive, widely available, and safe for both mother and child. The ultrasonographic approach to the evaluation of the fetal brain is based on the transabdominal visualization of three different axial planes: the transventricular, the transthalamic, and the transcerebellar (Figure 29-1). The use of these three planes enables good visualization of the lateral ventricles but is not sufficient for depiction of malformations of the cortex and of midline brain structures, mainly the corpus callosum, the third ventricle, and the cerebellar vermis [Malinger et al., 2007]. The use of a more comprehensive, multiplanar approach, in which coronal and sagittal planes are added to the classical axial planes, and addition of a transvaginal or transfundal approach overcome these limitations [Malinger et al., 1993] (Figure 29-2 and Figure 29-3).
Fetal MRI is used primarily to confirm and characterize brain abnormalities detected by routine prenatal sonography. More than 25 years after its introduction into fetal imaging, MRI is considered a valuable complementary tool in the imaging of structural anomalies of the fetal brain [Guibaud, 2009; Sonigo et al., 1998].
Fetal MRI is performed primarily using ultrafast MRI techniques known as single-shot, fast spin-echo (SS-FSE) or half-Fourier acquired single-shot turbo spin-echo (HASTE). Using these rapid-pulse sequences, a single T2-weighted image can be acquired in less than 1 second, reducing the likelihood of fetal motion during image acquisition [Brugger et al., 2006; Glenn and Barkovich, 2006]. When the fetal brain is being evaluated, images are obtained with a section thickness of 3 mm with no gap, in axial, sagittal, and coronal planes. Good orthogonal planes may not always be obtainable.
T1-weighted imaging and fast multiplanar gradient recalled-echo techniques, such as FMPSPGR (fast multiplanar spoiled gradient recalled acquisition in the steady state), are primarily used to detect hemorrhage or calcification [Glenn and Barkovich, 2006].
With the recent application of advanced MRI techniques, an opportunity to investigate the developing brain is available beyond the qualitative morphologic evaluation [Limperopoulos and Clouchoux, 2009; Glenn, 2009]. Diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) are used to evaluate maturation-dependent microstructural changes associated with brain growth and development of cerebral white matter. DWI provides quantitative information about water motion and tissue microstructure, and provides additional applications for destructive brain processes. DTI allows the visualization and quantification of white-matter fiber direction. Advanced postprocessing techniques, such as the formation of high-resolution three-dimensional structural images, can be used to study morphometry [Glenn, 2009]. MR spectroscopy can be applied in the third trimester for the study of cerebral metabolism in vivo, since metabolites such as N-acetyl aspartate, creatine, and choline can be detected [Limperopoulos and Clouchoux, 2009].
Prenatal Assessment of Normal Brain Development in the First Trimester
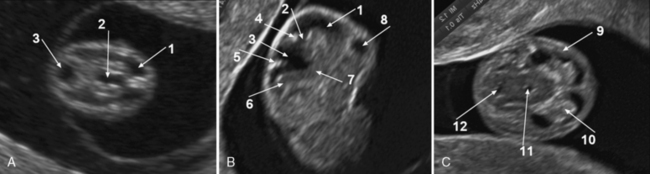
Brain development in the embryonic phase is only assessed by ultrasound. Blaas and Eik-Nes [2009] describe the milestones of normal brain development between 7 and 12 weeks’ gestation based on longitudinal two-dimensional and three-dimensional ultrasound studies. Cortical development starts at about 7 weeks’ gestation and other brain structures develop synchronously with the cortex, including the commissures and the cerebellum. At 7–8 weeks, the lateral ventricles are seen as separated, small, round hypoechogenic brain cavities, and the future third ventricle runs posteriorly. The curved tube-like mesencephalic cavity (future sylvian aqueduct) lies anteriorly, and the broad and shallow rhombencephalic cavity is in the cranial pole of the embryo (Figure 29-4).
By the end of the first trimester (weeks 10–12), the cortex is about 1–2 mm thick and the crescent hemispheres fill the anterior part of the head and conceal the diencephalic cavity. The lateral ventricles are large and filled by the echogenic choroid plexuses. The insula appears as a slight depression on the lateral surface of the hemispheres. The diencephalon lies between the hemispheres, and the mesencephalon gradually moves towards the center of the head. The width of the third ventricle narrows. The corpus callosum is not yet visible. The cerebellar hemispheres seem to meet in the midline [Blaas and Eik-Nes, 2009].
Prenatal Assessment of Normal Development of the Cortex
Initially, the brain surface is smooth. Gyration can be observed by the second month of intrauterine life. It continues to the end of the pregnancy and even later after birth. The primary sulci appear as shallow grooves on the surface of the brain that become progressively more deeply infolded and then develop side branches, designated secondary sulci. Gyration proceeds with the formation of other side branches of the secondary sulci, referred to as tertiary sulci [Nadich et al., 1994]. The major fissures and sulci develop in predictable patterns, starting at about 16 weeks [Dorovini-Zis and Dolman, 1997], and therefore the timing of their appearance is a reliable estimate of gestational age. Anatomical changes precede ultrasound and MRI detection by about 2 weeks [Levine and Barnes, 1999] (Table 29-1).
Table 29-1 Chronology of Sulcation According to Neuropathological, Sonographic, and Magnetic Resonance Imaging Studies
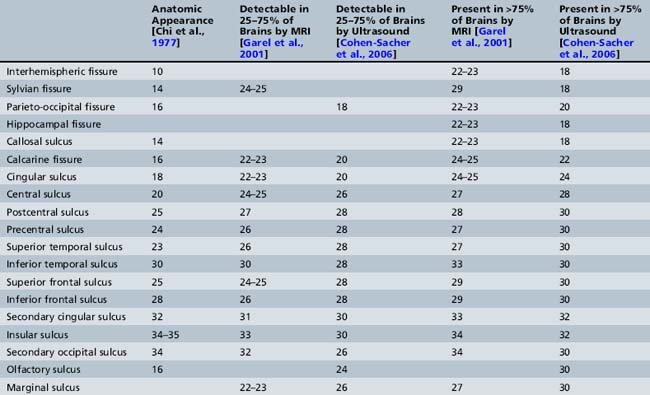
Several studies [Toi et al., 2004; Cohen-Sacher et al., 2006] have assessed the development of the fetal cortex by ultrasound. Major sulci can be discerned, starting at about 18–19 weeks’ gestation. At 18–20 weeks, the brain surface is almost completely smooth, with only the major fissures present. The callosal sulcus is observed between 18 and 20 weeks. Between 22 and 26 weeks, the calcarine fissure, the cingulate sulcus, and the central sulcus gradually appear. The most active period of cortical development, as demonstrated by ultrasound, is between 28 and 30 weeks. During this time, most sulci become detectable. After 32 weeks, all structures, other than the insular and olfactory sulci, are present. During the last 4 weeks of pregnancy, the sulci over the convexity are best demonstrated using sagittal planes [Cohen-Sacher et al., 2006] (see Table 29-1). Early sulci are easier to evaluate by ultrasound than later-developing complex secondary and tertiary patterns [Malinger et al., 2006].
MRI after about 20 weeks of gestation provides excellent information unimpeded by calvarial calcification. Similar to ultrasound, it shows age-related predictable patterns of development, but, in addition, brain parenchyma and myelination can be evaluated [Garel et al., 2001].
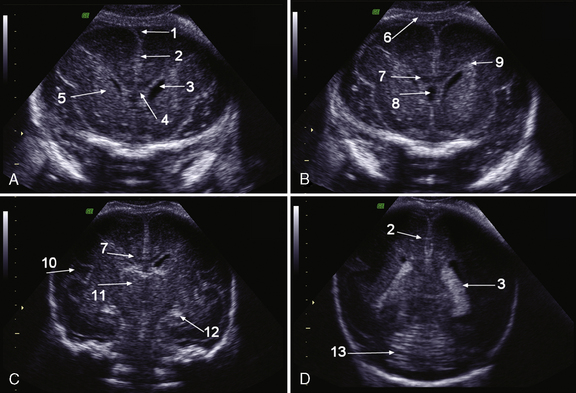
Brain MRI can depict the walls of the ventricles, which are critical to normal brain development. They are lined by the ventricular zone (germinal matrix), which is very thick earlier in gestation, and appears as a smooth band of dark T2 signal and bright T1 signal lining the lateral ventricles. The cerebral mantle is seen on fetal MRI until about 28 weeks of gestation in a multilayered pattern [Kostovic and Vasung, 2009]. When viewed from the ventricular margin to the pial surface of the brain, five layers can be demonstrated (Figure 29-5).
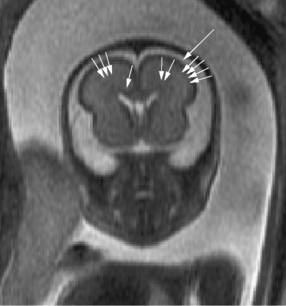
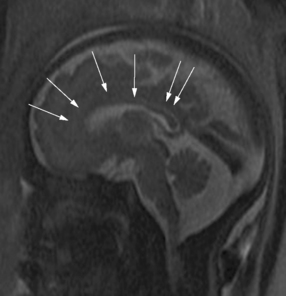
The development of the cortex can be visualized from midpregnancy. On MRI, gyration starts with the appearance of a shallow indentation of the fetal brain in the temporal regions at the 18th gestational week. At the same time, the anterior cingular fissure and the parieto-occipital fissure occur. The central sulcus begins to be visible at 24 weeks’ gestation. Fissures then become deeper and tighter, and gyri bulge into the subarachnoid space. Until the 35th gestational week, all primary and most of the secondary sulci are present, with secondary sulci appearing from the 24th gestational week, and tertiary after the 28th gestational week [Garel et al., 2001; Prayer et al., 2006] (see Table 29-1; Figure 29-6).
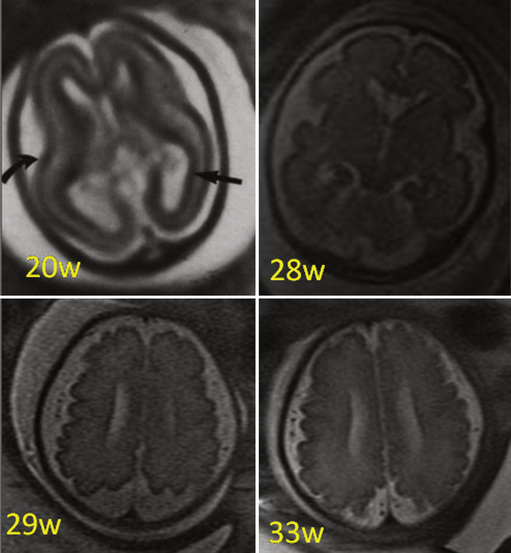
Fig. 29-6 Magnetic resonance imaging: axial images at different weeks of gestation.
Normal development of sulcation and normal “closure” of the insula are demonstrated.
The development of the sylvian fissure is one of the major brain maturational processes occurring in fetal life, and abnormalities in this process are frequent in children with developmental delay. The appearance and shape of the sylvian fissure can be evaluated both by ultrasound and MRI [Lerman-Sagie and Malinger, 2008; Garel et al., 2001; Chen et al., 1995]. It is observed in all fetuses at 22–23 weeks’ gestation as a shallow depression at the surface of the brain, and the insula is wide open at this time. The temporal lobe folds over to engulf the insula, and the progressive closure begins posteriorly and extends anteriorly. By 33 weeks’ gestation, insular sulci are recognizable and the surface of the Sylvian fissure becomes more and more indented [Garel et al., 2001; Quarello et al., 2008].
Prenatal Assessment of Normal Development of the Corpus Callosum
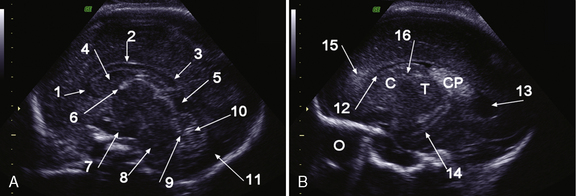
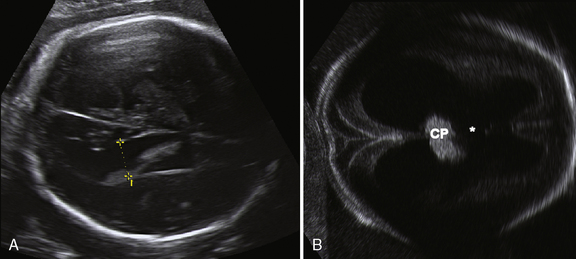
The corpus callosum develops between gestational weeks 8 and 20 as axons from the developing cerebral hemispheres navigate to and through the hemispheres and interhemispheric fissure. The corpus callosum is difficult to visualize by ultrasound during the performance of routine transabdominal evaluations using axial planes. For a complete demonstration of this structure, it is necessary to obtain sagittal planes through the anterior or posterior fontanels or the sagittal suture with the transvaginal or transfundal approach. Coronal planes are useful for studying the relationship between the corpus callosum and neighboring structures. The completely formed corpus callosum may be depicted using high-resolution transducers by 18–20 weeks of gestation, as an arched hypoechogenic structure delimited by the more echogenic callosal sulcus cranially and the anechogenic cavum septi pelucidi caudally. At this age, its shape is already very similar to that of the mature corpus callosum, and the genu, body, and splenium can be well demonstrated (see Figures 29-2 and 29-3). The corpus callosum develops in a linear pattern during pregnancy, its length progressively increasing from 20 mm at 20 weeks to 40 mm close to term. Nomograms of callosal growth during pregnancy, including length and thickness, can be used to assess normal development [Malinger et al., 1993].
The corpus callosum can be depicted by MRI on midline sagittal T2 images by gestational week 20 as a band of low signal intensity superior to the fornix (Figure 29-7). It can also be seen on coronal T2 images. It has fairly uniform thickness [Toi et al., 2009].
Prenatal Assessment of Normal Development of the Posterior Fossa
Both ultrasound [Malinger et al., 2001] and MRI [Glenn, 2009; Adamsbaum et al., 2005] can evaluate the development of the fetal cerebellum.
The ultrasonographic evaluation should include multiplanar images of the cerebellum. The axial plane is useful for determining the transcerebellar diameter, cisterna magna size, and the cerebellar peduncle’s thickness. The coronal plane enables differentiation between the cerebellar hemispheres and the vermis. The midsagittal plane is the most important plane to be investigated, since it allows depiction of the vermian lobules, fissures, and shape of the fastigium; measurements of the vermian diameter and surface; and calculation of the ratio between the superior and inferior parts. This plane also permits evaluation of the size and shape of the pons, cisterna magna, and tentorium [Malinger et al., 2001].
The fourth ventricle is uniformly observed as a triangular structure anterocaudal to the vermis. The inferior lobe of the vermis and the nodulus separate the fourth ventricle and the cistern magna [Malinger et al., 2001].
MRI is highly accurate in illustrating the morphologic MRI biometry of cerebellar development [Adamsbaum et al., 2005; Triulzi et al., 2005]. The cerebellar vermis is best assessed by MRI on direct midline sagittal images and on coronal images; the measurements should be compared with established norms. The cerebellar hemispheres are best assessed on nonoblique axial and coronal views.
Fetal MRI shows gestational age-specific changes in signal intensity in the normal development and maturation of the cerebellar hemispheres and brainstem [Huisman et al., 2002]. The cerebellar cortex, dentate nucleus, tectum, dorsal pons, and medulla are T1-hyperintense and T2-hypointense. The changes in signal intensity in the brainstem and cerebellum are not encountered until 20–23 weeks of gestation. By 26–27 weeks of gestation, a three-layered pattern is noted in the cerebellar hemispheres, corresponding to the cerebellar cortex, cerebellar white matter, and dentate nucleus. Fetal brain MRI can show the fissures of the cerebellum, depending on the gestational age. The primary fissure is identified on sagittal images at 22 weeks but the cerebellar surface is smooth. From 24 to 29 weeks, foliation of the vermis and posterior lobes of the cerebellum is seen on sagittal images. The cerebellar surface is smooth, with the appearance of some indentations corresponding to the horizontal and secondary fissures on axial images. The convoluted pattern of the cerebellum is well identified from 30 weeks on and is always seen beyond 33 weeks. [Fogliarini et al., 2005a].
Posterior fossa anatomy is sufficiently well defined in order to diagnose most cerebellar abnormalities prenatally; however, there may be many pitfalls in the differentiation of normal and abnormal cerebellar development [Malinger et al., 2009; Limperopoulos et al., 2008].
Prenatal Diagnosis of Ventriculomegaly
The lateral ventricle should be measured by ultrasound in the axial plane, at the level of the frontal horns and cavum septi pellucidi, with the calipers positioned at the level of the internal margin of the medial and lateral wall of the atria, at the level of the glomus of the choroid plexus, on an axis perpendicular to the long axis of the lateral ventricle [ISUOG guidelines, 2007].
Ventriculomegaly is defined as a lateral ventricular width equal to or larger than 10 mm [ISUOG guidelines, 2007]. Fetal ventriculomegaly may be classified as mild when the lateral ventricular width is between 10 and 15 mm, and severe when larger than 15 mm; it may be unilateral or bilateral (Figure 29-8).
Ventriculomegaly is defined as isolated if there is no sonographic evidence of associated malformations or markers of aneuploidy at the time of the initial presentation. Isolated mild ventriculomegaly represents a diagnostic and counseling difficulty, as it can be an apparently benign finding, but can also be associated with chromosomal abnormalities, congenital infection, cerebral vascular accidents or hemorrhage, and other fetal cerebral and extracerebral abnormalities; it may also have implications regarding long-term neurodevelopmental outcome [Melchiorre et al., 2009]. Therefore, upon confirmation of ventriculomegal, a complete search for associated central nervous system and non-central nervous system anomalies should be attempted, including a study of the brain in a multiplanar approach with particular attention to the shape of the ventricles, periventricular white matter, sulcation, corpus callosum, and posterior fossa [Malinger et al., 2006]. The investigation should also include screening for in utero infection, amniocentesis, and fetal echocardiography. Many studies have indicated that MRI may add important information to that obtained by ultrasound imaging [Ouahba et al., 2006]. Information relevant enough to modify obstetric management can be obtained in 6–9 percent of cases, including cortical malformations, absence of the septum pellucidum, partial agenesis of the corpus callosum, and agenesis of the cerebellar vermis [Salomon et al., 2006; Valsky et al., 2004].
Follow-up examinations at 3–4-week intervals are indicated to assess progressive enlargement of ventricles or to diagnose associated pathologies not previously detected [Ouahba et al., 2006].
When mild ventriculomegaly is isolated, the outcome is usually good [Ouahba et al., 2006; Melchiorre et al., 2009]. However, the risk for abnormal outcome increases when there are associated anomalies, the ventriculomegaly is asymmetric, the atrial width is greater than 12 mm, or there is a progressive increase of the lateral ventricular width [Pilu et al., 1999; Sadan et al., 2007; Falip et al., 2007]. A false-negative diagnosis of associated anomalies is possible in up to 12.8 percent of cases [Melchiorre et al., 2009].
The outcome of severe ventriculomegaly depends mainly on the presence of associated pathologies. When associated pathologies are diagnosed, the prognosis is usually very poor. Even when isolated, the risk of perinatal death or severe neurologic sequelae is in the range of 50 percent of survivors [Kennelly et al., 2009].