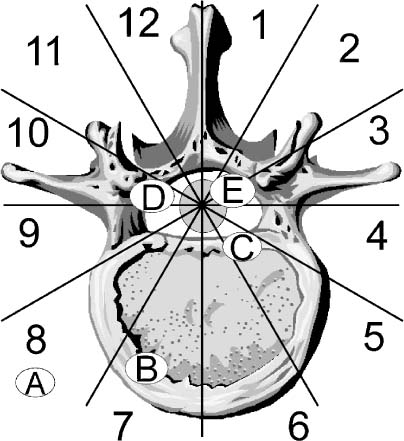
23 Primary tumors of the vertebral column are uncommon. In large published series, primary vertebral column neoplasms account for only 11% of all primary musculoskeletal tumors,1,2 4.2% of vertebral column tumors,3 and 0.4% of all neoplasms.4 They are ~40 times less common than spinal column metastases, which account for the vast majority of neoplasms in this anatomic locale. This is both a reflection of the prevalence of systemic neoplasms arising in the lung, breast, or prostate as well as these cancers’ predilection for spread to the vertebral column. Because of these tumors’ uncommon nature, spinal surgeons are likely to encounter few primary spinal column tumors in day-to-day practice. As such, these tumors often receive only secondary consideration in the diagnostic evaluation of spinal lesions; however, to ensure that patients harboring a primary vertebral column neoplasm receive a treatment plan that correctly utilizes modern spinal surgical techniques, surgeons must consider this class of lesions in their differential diagnoses. This chapter provides the reader with an understanding of the clinical presentation, natural history, and viable treatment options for the many tumors within this broad category. The majority of patients harboring a primary vertebral column tumor present with nonspecific neck or back pain, which likely accounts for the often lengthy period between initial presentation and definitive diagnosis of primary spine tumors. Despite the nondescript pain associated with primary bone tumors, there are several characteristics that can help to differentiate the pain of these lesions from that of muscular, fascial, discogenic, spondylolytic, or inflammatory pain. The pain associated with vertebral axis neoplasms is classically described as unremitting, worse in the supine position, less relieved by positional changes, unresponsive to analgesia, and more noticeable while at rest or in bed at night.5 Radicular pain secondary to compression or invasion of nerve structures is rare. The majority of tumor pain is localized within the vertebral axis secondary to neoplastic infiltration of bony structures or due to mechanical instability following neoplastic destruction of one or more facet joints. Moreover, the majority of conditions that cause neck, back, or radicular pain, such as degenerative disease, disc herniation, and spondylosis, are uncommon in young patients. Thus, primary vertebral column lesions should be highly considered as the etiology of axial pain in children and young adults. Primary vertebral column neoplasms may also present as a spinal deformity (with or without pain). Pediatric patients with osteoid osteomas and osteoblastomas frequently present with torticollis, scoliosis, or kyphosis.6,7 Thus, in cases of new-onset deformity the diagnosis of an occult spinal neoplasm should be pursued even if screening radiographs are normal. The expediency of diagnosis is imperative in such cases, as an inverse relationship exists between the time delay from symptom onset to tumor extirpation and the likelihood of resolution of deformity.6 Patients may also present with a painless neurologic deficit (radiculopathy or myelopathy), as a painless localized mass extending from the posterior elements of the spine into the paraspinal musculature, or as an incidental finding on an imaging study. In considering the differential diagnosis of vertebral axis lesions, both the age of the patient and the localization of the lesion are important. In general, patients with benign lesions tend to be younger, with an average age of presentation of 20 to 30 years, whereas those with malignant primary and metastatic spinal lesions present after 40 years of age.5 A disproportionately low number of primary tumors are located in the cervical spine, in comparison with the thoracic and lumbar regions,3,5,8 whereas vertebral body involvement occurs in 80% of malignant lesions and only 40% of benign lesions.9 The diagnosis of a primary vertebral axis neoplasm requires detailed imaging of the region of the spine in question. Although modern neuroimaging methods have greatly facilitated the diagnosis of these lesions, the plain radiograph remains a useful first test. Spine radiographs should be obtained for patients with persistent back pain, new-onset deformity, or neurologic deficits, as well as for all children with back pain. Radiographs reveal an abundance of information, including vertebral collapse, kyphosis, and subluxation, as well as neoplastic destruction of bone; however, as 60% of bone must be replaced by neoplasm before lesions become visible on plain radiographs,10 a normal radiograph should not dissuade one from pursuing the diagnosis of a vertebral lesion with more advanced imaging modalities when the history and physical examination favor such a diagnosis. Given the inherent complex anatomy of the vertebral axis, further characterization of the lesion and the extent of vertebral axis involvement is best performed with imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI). CT scanning is best utilized to provide information about cortical bone and mineralized matrix,11 whereas MRI is superior in delineating epidural and bone marrow tumor infiltration and distinguishes the extraosseous soft tissue component of a neoplasm from the normal paraspinal soft tissue and neural structures.11,12 MRI, particularly T2- weighted images, is highly sensitive to the presence of signal abnormalities in the spinal cord resulting from neoplastic compression.12 In those with contraindications to MRI, CT-myelography provides excellent visualization of the thecal sac and can demonstrate spinal canal compromise by invading neoplasm. Individuals suspected of having either multifocal primary or metastatic disease should also undergo a technetium bone scan. It is invaluable in quantifying the extent of bone involvement over the entire skeletal structure and provides information about bone forming and bone destroying neoplastic entities. As many primary vertebral tumors are vascular, spinal angiography should also be routinely performed to assess the need for preoperative tumor embolization. Angiography is an especially useful preoperative investigation for lesions of the lower thoracic or upper lumbar spine, as identification of the artery of Adamkiewicz greatly aids preoperative planning. Lesional biopsy is the last step in the evaluation of a vertebral axis neoplasm and should be performed only after imaging studies have delineated both the local and systemic extent of the lesion. There are three types of biopsy techniques for suspected vertebral axis neoplasms: (1) fine-needle aspirate (FNA) biopsy, (2) incisional biopsy, and (3) excisional biopsy. The vast majority of spinal lesions are appropriately biopsied using either FNA or incisional biopsy, whereas excisional biopsy should be reserved for posterior lesions in which the differential diagnosis is limited to benign lesions.13 CT-guided FNA has become the most common biopsy technique for suspected spinal neoplasms, as it couples lower complication rates with reduced extralesional spread of tumor cells9,14 and provides a tissue diagnosis in 70 to 80% of attempts.14,15 Careful selection of the biopsy method, as well as the specific anatomic route, is critical to the overall management of patients with vertebral axis lesions. Once the tumor mass and surrounding normal tissue have been violated, all other treatment options become dependent on this initial invasive procedure.16 To avoid seeding of healthy tissue with neoplastic cells, it is imperative that the biopsy route be planned so that it can be excised with adequate margins at the time of definitive resection. Following this tenet, biopsy of vertebral body lesions, whether performed open or percutaneously, should be approached anteriorly or via a transpedicular approach to avoid contamination of the epidural space, as occurs during laminectomy. To plan an appropriate and definitive surgical treatment for a vertebral axis lesion, the spine surgeon must stage the neoplasm both oncologically and surgically. Staging involves the integration of information regarding the anatomic localization, histologic grade, and presence or absence of metastases in an attempt to predict the biologic behavior of the lesion. Enneking2 developed an oncologic staging system for musculoskeletal tumors that is effective in guiding surgical therapy for limb lesions (Table 23-1). This system has been successfully applied to primary spinal column tumors. 9,17 Benign tumors are divided into three stages: S1, inactive; S2, active; and S3, aggressive. S1 lesions are not growing, are asymptomatic, and are surrounded by a well-defined capsule easily seen on plain radiographs. S1 lesions can be treated conservatively unless surgery is needed for spinal decompression or stabilization. S2 benign tumors are slow-growing lesions associated with mild symptoms. A thin capsule as well as a reactive tissue pseudocapsule surround the tumor. The majority of S2 lesions are appropriately managed with intralesional excision or curettage. S3 tumors are rapidly growing lesions that have a thin or absent capsule and that have invaded the epidural space or neighboring soft tissues. A wide hypervascular pseudocapsule is usually present. Surgical management of S3 lesions should include an attempt at en bloc excision, as intralesional excision is associated with a high rate of recurrence.18 Adjuvant radiotherapy is often indicated. The Enneking system also stages malignant tumors, based on tumor histology. Subclassification is based on anatomic compartmentalization, with A lesions defined as intracompartmental (within the vertebrae) and B lesions defined as extracompartmental (invading paravertebral structures. Low-grade malignant tumors (stages IA and IB) have no true capsule but often have a thick pseudocapsule that is penetrated by islands of tumor. The treatment of choice, if feasible, is wide en bloc excision followed by adjuvant radiotherapy.19 High-grade malignant tumors (stages IIA and IIB), because of their rapidity of growth, have no reactive pseudocapsule. In addition, neoplastic nodules are often found at some distance from the main tumor mass. Because of the difficulty in achieving radical resection margins in the vertebral column, surgical results are less gratifying. Stage II lesions should be treated with as wide a resection as feasible, followed by adjuvant chemotherapy and/or radiotherapy, depending on tumor type. All malignant lesions associated with distant metastasis are classified as Enneking stage III. Palliation should be the goal of treatment for these lesions. Surgical staging occurs after the diagnosis has been established and the oncologic staging complete. The unique anatomy of the spinal column and the restrictions that the contents of the thecal sac place on surgical resection have mandated the formulation of surgical staging systems specific to the spine. The most widely used is the Weinstein-Boriani-Biagini (WBB) system. This system has been validated for both benign and malignant vertebral column tumors.17,19 This system divides the vertebrae into 12 radiating zones, moving in a clockwise fashion, with zone 1 at the spinous process, and five layers (A to E) in the transverse plane (Fig 23-1). The WBB system allows for a rational and logical approach to surgical planning, taking into account the limitations to marginal en bloc excision that exist in the spinal column. For instance, en bloc tumor excision can be performed if the tumor is confined to WBB zones 4 to 8 or 5 to 9; that is, a centrally located lesion with at least one pedicle free of tumor. FIGURE 23-1 The Weinstein-Boriani-Biagini (WBB) surgical staging system for primary vertebral column tumors. The spine, neural canal, thecal sac, and surrounding soft tissues are divided into 12 numerical zones and six lettered compartments to allow precise staging and decision making regarding the most appropriate operative approach. Layer A represents extraosseous soft tissue, B is superficial intraosseous, C is deep intraosseous, D designates extradural tumor extension, and E signifies intradural tumor spread. The axial skeleton is composed of an outer cortical frame of dense bone and internal network of cancellous bone surrounding hematopoietic red marrow (in the vertebral body) and yellow marrow (in the posterior elements). Associated with the osseous framework of the vertebral axis are cartilaginous and fibrous elements, whereas notochordal remnants are also located along the length of the vertebral column. Benign and malignant neoplasms may arise from any of these tissue elements. Thus, primary vertebral axis lesions can be conveniently categorized based on their tissue of origin (Table 23-2). Although benign primary vertebral column neoplasms encompass a wide range of histologic types, this group of lesions still shares many common features. The majority present secondary to localized pain or spinal deformity, although neurologic deficits occur with increasing frequency as the aggressiveness of the lesion increases. Due to the complex anatomy of the spinal column, the diagnosis of small benign lesions may not be possible using plain radiographs. Judicious use of adjunct diagnostic imaging modalities such as CT, MR, and technetium-99m bone scanning are crucial to visualize many benign vertebral column lesions. Given the multiplicity of tumor histology, oncologic staging of benign lesions is imperative to guide therapeutic decisions. Internal curettage is an appropriate surgical strategy for benign tumors only when the lesion is well contained within bone and has little propensity for recurrence. Marginal en bloc excision is prudent for more aggressive lesions but cannot often be accomplished, given the anatomic constraints of the vertebral column. As such, radiotherapy and chemotherapy should be considered in the management of those aggressive benign lesions that have recurred or whose location prevents adequate marginal resection.
Primary Vertebral Column Tumors
 Clinical Presentation
Clinical Presentation
 Diagnostic Imaging
Diagnostic Imaging
 The Role of Biopsy
The Role of Biopsy
 Oncologic and Surgical Staging of Tumors
Oncologic and Surgical Staging of Tumors
 Classification of Primary Vertebral Column Tumors
Classification of Primary Vertebral Column Tumors
Benign Neoplasms
| Tissue of origin | Benign | Malignant |
| Osseous | Osteoid osteoma* | Osteosarcoma* |
| Osteoblastoma* | ||
| Cartilaginous | Osteochondroma* | Chondrosarcomas* |
| Hematopoietic | Solitary plasmacytoma* | |
| Ewing’s sarcoma* | ||
| Lymphoma | ||
| Multiple myeloma | ||
| Fibrous | Fibroma | Fibrosarcoma |
| Malignant fibrous histiocytoma | ||
| Vascular | Hemangioma* | |
| Aneurysmal bone cyst* | ||
| Notochordal | Chordoma* | |
| Other | Giant cell tumor* | |
| Eosinophilic granuloma* | ||
| Brown tumor* |
*Discussed in detail in the text.
Osteoid Osteoma
Osteoid osteomas are benign osteogenic tumors found predominantly in adolescents or young adults, with a 2:1 male predominance. Pain is the most common presentation and is characteristically worse at night and dramatically relieved with salicylates or nonsteroidal antiinflammatory medications.20 Other common presenting symptoms include pain-limiting range of motion of the spine, as well as painful torticollis or scoliosis.20 Osteoid osteoma is the most frequent cause of painful scoliosis in adolescents and is considered to be secondary to pain-provoked muscle spasm ipsilateral to the lesion. A large case review noted that 63% of patients with spinal osteoid osteomas had scoliosis.21 Osteoid osteomas have a predilection for the posterior elements of the spine (Fig 23-2), with 75% located posteriorly and only 7% in the vertebral body.22
Radiologically, this lesion consists of a discrete radiolucent nidus surrounded by a variable degree of sclerosis. The complex anatomy of the spine coupled with a variable degree of sclerosis frequently makes osteoid osteomas undetectable by plain radiographs. As such, CT is the modality of choice, as it can confirm the exact anatomic location within the vertebrae and shows the nidus as a well-defined, low-attenuation lesion, with or without central calcification.23 Bone scans can also be used to define the lesion and demonstrate increased radionuclide uptake surrounding a “cold” nidus. Histologically, osteoid osteomas are identical to osteoblastomas and are composed of well-organized, interconnected trabecular bone around a nidus of vascularized fibrous connective tissue (Fig 23-2). The nidus of an osteoid osteoma is less than 1.5 cm in diameter; larger lesions are defined as osteoblastomas. Reactive bone invariably forms a pseudocapsule around the lesion.
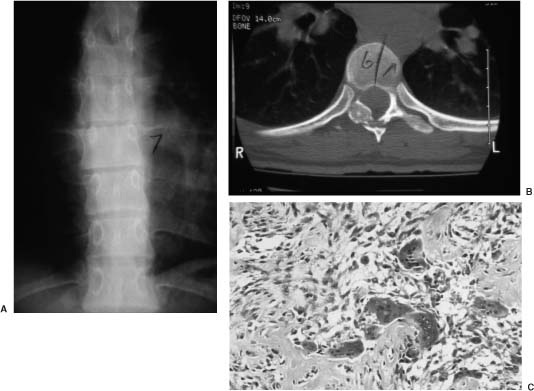
FIGURE 23-2 This 22-year-old woman presented after several years of unremitting back pain that had been initially responsive to nonsteroidal antiinflammatory medications. Plain x-rays (A) and computed tomography (CT) (B) reveal an ovoid, well-circumscribed lesion with peripheral sclerosis and a radiolucent center that involves the right lamina and pedicle at T7. The lesion was treated by complete excision. (C) Histologically, the lesion was characterized by the presence of osteoblasts and osteoid material against the background of a highly vascularized stroma, consistent with an osteoid osteoma. After surgery, the patient’s symptoms resolved and the lesion has not returned.
Surgical excision by intralesional curettage is the treatment of choice for these benign tumors. Anatomic localization is the greatest challenge at surgery, as morphologically osteoid osteoma is very similar to normal cortical bone. This similarity also makes it difficult to determine when total surgical extirpation has been achieved. Numerous strategies, including preoperative radioactive labeling of the nidus or CT-guided needle placement, have been employed to ameliorate these problems. Fusion is rarely required due to their inherently small size. Recurrence is unlikely if the lesion has been totally removed. Persistent pain after removal generally implies subtotal lesional excision. Complete excision results in pain relief and spontaneous deformity correction, especially if the deformity has been present for less than 15 months.6,20 There is no indication for radiation therapy in the treatment of osteoid osteoma.
Osteoblastoma
Osteoblastomas account for ~1% of all primary bone tumors and 10% of all osseous tumors of the spine.24 The vertebral column is their most frequent site of occurrence, accounting for 32 to 45% of osteoblastomas.7,18,24 Osteoblastomas occur with equal frequency in the cervical, thoracic, and lumbar segments of the spine. Vertebral axis osteoblastomas occur more commonly in males, with a male-to-female ratio of 2.5:1. Like osteoid osteomas, osteoblastomas have a strong predilection for the posterior elements, with only 3% of lesions isolated to the vertebral body.7,24 Osteoblastoma is a tumor of the young, with 80% of cases presenting by 30 years of age.7 Pain, especially at night, is the most common presentation. Unlike osteoid osteoma, the pain associated with osteoblastoma is relieved with salicylates in only a small fraction of cases.7 Although scoliosis and torticollis are more commonly associated with osteoid osteoma, osteoblastomas can also present secondary to spinal deformity. Scoliosis is especially common in association with thoracic and lumbar lesions.7,21 Neurologic deficit is more common with osteoblastomas than osteoid osteomas, occurring in up to 30% of cases,7,18 and is attributable to their larger size and more invasive nature.
The plain radiographic and CT appearance of osteoblastoma is more heterogeneous than that of osteoid osteoma. Although osteoblastomas can appear similar to osteoid osteomas, they are usually more geographically expansile lesions with or without calcification. Occasionally, osteoblastomas may appear aggressive, with bony destruction and soft tissue infiltration.24,25 The MR appearance of osteoblastoma is nonspecific, with low to intermediate T1 signal and intermediate to high T2 signal and variable gadolinium enhancement.26 Bone scintigraphy remains the most sensitive test, demonstrating intense radionuclide uptake, and in some cases may in the face of symptomatic lesions.27
Histologically, osteoblastomas are usually indistinguishable from osteoid osteomas and consist of trabecular bone and a fibrovascular stroma with numerous benign-appearing osteoblasts. Osteoblastomas may occasionally contain more prominent epithelioid osteoblasts and appear more histologically aggressive. Despite this appearance, the histology has not been found to be an accurate predictor of clinical behavior.24 Transformation to osteosarcoma is very rare and is typically seen only in the setting of multiple recurrences.24
Surgical resection, either by internal curettage or marginal en bloc excision, is the mainstay of treatment for vertebral osteoblastomas. The larger size of osteoblastomas occasionally necessitates destabilizing the spine to achieve complete excision.18 Obviously, in such cases, spinal instrumentation and fusion are required. Despite complete surgical resection, recurrence rates are somewhat higher than with osteoid osteomas and approach 10%.24 Although there is little data on the efficacy of adjuvant oncologic therapies, chemotherapy and radiotherapy, either alone or together, can be used in selected patients with recurrent tumors or surgically unresectable disease.28
Osteochondroma
Osteochondromas account for 30 to 40% of all benign osseous lesions,29 but only 4% occur in the vertebral axis, making this entity an uncommon primary vertebral column tumor.29,30 Although most vertebral osteochondromas are solitary lesions, multiple lesions can occur, usually in the setting of the autosomal-dominant genetic disorder hereditary multiple exostoses (HME).31,32 These benign cartilaginous tumors are thought to arise during development when rests of cartilage become trapped outside the advancing epiphyseal growth plate.
Vertebral osteochondromas form as a sessile or pedunculated stalk associated with a bony base and a cartilaginous cap (Fig 23-3). They have a predilection for the cervical spine (>50% of vertebral osteochondromas), particularly C2. This propensity may be associated with the greater mobility of the cervical spine, which, in turn, leads to increased stress and microtrauma on cartilaginous plates. This results in trapping of cartilage outside of the physeal plate.33 Reflecting their developmental origin, osteochondromas are usually found in patients less than 30 years of age,29 with HME-associated tumors presenting ~10 years earlier.32 There is a male predominance, greater than 2:1 in non-HME cases and slightly less in HME cases.29 Osteochondromas are more commonly found in the posterior elements of the vertebrae.29 Rarely, osteochondromas can undergo malignant transformation to chondrosarcomas.34 Although osteo chondromas may present secondary to localized pain, neurologic complications are also possible secondary to their expansile growth into the spinal canal. Myelopathy is the presenting symptom in 34% of patients with solitary lesions and 77% of HSE-associated osteochondromas. 29 Anteriorly located cervical lesions may rarely present secondary to dysphagia or vascular compression.35
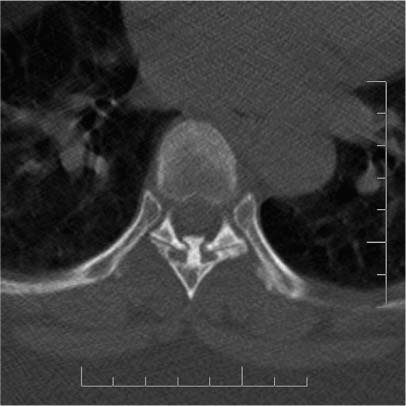
FIGURE 23-3 Axial CT through T7 reveals the typical radiographic appearance of osteochondroma. This pedunculated lesion and its associated cartilaginous cap were resected via laminectomy.
Histologically, osteochondromas are composed of normal bone with a cartilaginous cap from which the growth occurs. The pathologic hallmark is the continuity of the lesion with the marrow and cortex of the underlying bone. Thin-section CT is the imaging modality of choice, as it can detect the pathognomonic marrow and cortical continuity of this lesion with the underlying bone.23 MRI can also offer good resolution of the marrow, osseous, and cartilaginous components of the tumor. The hyaline cartilaginous cap has intermediate signal on T1-weighted images and high signal on T2 images. In an adult, cartilaginous caps greater than 1.5 cm in thickness suggest malignant degeneration to a chondrosarcoma.11
Asymptomatic lesions can be followed conservatively, but surgery should be considered whenever the diagnosis is in question, if there is pain, or in cases of neurologic deficit.29,30,36 Due to their predilection for the posterior elements, a posterior approach can be used for the majority of lesions. The recurrence rate is extremely low after en bloc excision or internal curettage, but documentation of regrowth after subtotal excision has been reported.29
Hemangioma
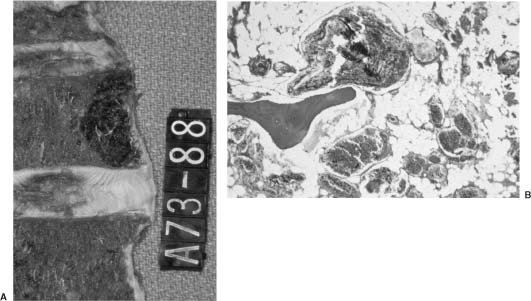
Hemangiomas are benign vascular tumors. They are the most common neoplasm affecting the spinal column, found in 10 to 12% of spines at autopsy37 (Fig 23-4A). Hemangiomas may involve any portion of the spine but occur most commonly in the thoracic spine. Multiple noncontiguous levels may be involved. They usually involve the vertebral body but can be found in the posterior elements and, rarely, in all three vertebral columns.38 Although the majority of hemangiomas are asymptomatic, they can cause pain and localized tenderness secondary to bony expansion, epidural extension, vertebral collapse/pathologic fracture, or rarely, due to the effect of compression on neural structures. Clinical presentation peaks in the fifth decade. Rare cases of hemangioma symptomatology exacerbated by pregnancy, due to either hormonal or hemodynamic influences, have been documented.39,40
Hemangiomas have a very characteristic radiologic appearance on plain radiography and demonstrate hypertrophic vertical trabeculae, which occurs in response to the neoplastic destruction of horizontal trabeculae.41 Due to the high content of adipose tissue, hemangiomas are bright on T1 MRI. Slow flow within the prominent vascular components also results in bright signal on T2 MRI, making MRI very sensitive at detecting hemangiomas and in defining their epidural extent. Histologically, hemangiomas are composed of either large, irregular endothelial-lined spaces (cavernous type) or multiple blood vessels (capillary type). The former consists of dilated blood vessels without intervening bone stroma (Fig 23-4B) and is the most common variant, whereas the latter consists of thin-walled vessels separated by normal bone.
Vertebral hemangiomas that present due to local pain and tenderness are most appropriately treated by nonsurgical methods. Traditionally, external bracing has been a mainstay of treatment. Recently, modalities such as radiotherapy, vertebroplasty, and endovascular embolization have been used to treat symptomatic lesions. Although there is no definitive evidence attesting to the efficacy of these treatment modalities, anecdotal evidence suggests that they are safe and offer symptomatic relief. Radiotherapy to a total dose of 20 to 30 Gy was associated with symptomatic relief for two thirds of patients at 5 months after treatment in one series,42 whereas percutaneous vertebroplasty has also been shown to be safe and offer symptomatic relief in a significant portion of patients.43 Experience also suggests that endovascular embolization, a modality that has long been an invaluable adjunct to surgery, is also effiscacious as a stand-alone therapy in the treatment of hemangiomas.44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree