Principles and Techniques for Long-Term EEG Recording (EMU, ICU, Ambulatory)
Jean Gotman
Marc Nuwer
Ronald G. Emerson
INTRODUCTION
Most standard EEG recordings last 20 to 30 minutes and some are extended to 1 or 2 hours. It is also possible to record the EEG over long periods of time, from a few hours to days or weeks. This is the domain of “long-term monitoring” (LTM) and this chapter will discuss when such long recordings should be performed and in particular the special recording and analysis techniques that are available to facilitate the collection and review of these data in the most efficient way, while providing the patient with minimum discomfort. Most such LTM is performed in the context of episodic disorders and the characterization of the behavioral component of these disorders is an integral part of the diagnosis process. Video recording of the patient, synchronized with the EEG, is therefore an integral part of LTM.
Long-term EEG and video recording were born in the 1970s and became a mature field in the early 1980s (1). The technology has considerably improved since then but fundamental aspects have not really changed, with the possible exception of LTM in the intensive care unit (ICU), which has only developed in the last few years. It is interesting to note that the combined recording of EEG and behavior has a long history in the context of epilepsy (2); at that time the “long-term” part was missing and only the “EEG video” (albeit on film) was possible for a few minutes. The recording of behavior only became practically feasible over extended periods in the 1970s with time-lapse video recorders, recording one or just a few frames per second. Major leaps occurred with the availability of VCRs and more recently with the digital recording of video signals. With respect to the ability to record EEG signals over the long term, the storage capacity of computers has steadily increased, such that it was possible to record discontinuous samples in the 1970s and 1980s and then it became possible to record continuously for 24 hours in the 1990s, at the standard sampling rate of 200 Hz. Today the storage requirements for the long-term EEG signal have become trivial, except in the context of large numbers of channels (100 or more) and very high sampling rates (2000 Hz).
LONG-TERM MONITORING FOR EPILEPSY
Indications
The International League Against Epilepsy has published guidelines for the use of LTM in epilepsy (3). In order to make a solid diagnosis of epilepsy and of the type of epilepsy, a description of the behavioral and cognitive manifestations of the seizures is necessary. This is most often obtained from the patients and their relatives. In many cases, the description is incomplete or even erroneous: patients are often not aware of their seizures or of parts of their symptoms and relatives are frequently poor historians and poor observers. In some cases, it is important to know the EEG manifestations of the seizures in order to make a correct diagnosis. For example, behavioral arrest can occur with primary generalized epilepsy and generalized spike and wave and with a temporal lobe seizure. One can refine the indications for LTM as follows (3):
The diagnosis of epilepsy needs to be made or rejected, as the events cannot be unambiguously classified from historical information. Some patients have epileptic and nonepileptic seizures.
The diagnosis of epilepsy has been made but the type of seizure or syndrome is not clear.
Epilepsy is medically intractable and surgery is considered. This requires a full characterization of the electroclinical manifestations of seizures, at first with scalp EEG, and possibly subsequently with intracranial electrodes in case the scalp EEG does not provide sufficient information.
Seizures need to be quantified to assess response to medical treatment, as may be the case in brief but frequent seizures.
In addition, LTM allows recording throughout the sleep-wake cycle. Some patients have predominantly or exclusively seizures during sleep, others predominantly on awakening. Interictal activity in focal epilepsy is activated by non-REM sleep and in some patients is only present during that stage.
Equipment and Personnel
The basic components of a LTM system are the electrodes and electrode wires, the amplifier, the EEG transmission system, the camera, and the recording computer. We will examine the characteristics of these different components.
Electrodes
Electrode attachment is a particularly important aspect of obtaining good-quality EEG during LTM and while the patient experiences the many movements caused by the activities (e.g., eating) present during a recording that last several days and while the patient has seizures, which can include violent movements. Electrode problems are the most common cause of poor quality of a recording. The scalp electrodes must be placed in such a way that they provide a good contact for a long period, typically at least 24 hours, sometimes longer if technicians are not available every day or during weekends. Collodion or other durable electrode-scalp adhesive is therefore recommended. It is important to minimize artifacts caused by the movement of
electrode wires, which are particularly common during seizures. This can be achieved by wrapping a bandage or placing elastic netting over the patient’s head, thus preventing the wires from moving. This also limits the patient’s access to electrodes (e.g., to prevent dislodging electrodes during scratching). All wires can be bundled together until the input of the amplifier: the bundle has more rigidity than individual wires and will also limit movement artifact.
electrode wires, which are particularly common during seizures. This can be achieved by wrapping a bandage or placing elastic netting over the patient’s head, thus preventing the wires from moving. This also limits the patient’s access to electrodes (e.g., to prevent dislodging electrodes during scratching). All wires can be bundled together until the input of the amplifier: the bundle has more rigidity than individual wires and will also limit movement artifact.
If a patient undergoing LTM requires an MRI, the electrodes must be removed and then replaced after the study. This can require a considerable time. Electrodes that can be kept during MRI studies have been developed recently. They include MR-compatible material for the electrodes and the wires and an MR-compatible connector system. Such electrodes include gold, silver-silver chloride, subdermal silver wire, and conductive plastic electrodes (4,5). Some of these electrodes are also CT-compatible, greatly facilitating EEG monitoring of patients in the ICU.
It has been shown in multiple studies that electrodes recording from regions lower than the standard 10/20 electrodes are important in the characterization of temporal and inferior frontal discharges. A minimum configuration for LTM should therefore include 25 electrodes: the 19 electrodes of the 10/20 system plus three inferior electrodes on each side (e.g., F9, T9, P9, F10, T10, P10 of the 10/10 system). Recordings are most often referential and it is therefore particularly important to have a reference of good quality. Display with any other reference can be subsequently calculated. The EKG must also be recorded since cardiac changes can be an important component of seizures or seizure-like events. It may be useful in some situations to use additional electrodes in order to obtain better spatial sampling of a region. We will not discuss here the various types of intracerebral electrodes as this is addressed elsewhere in this volume.
EEG Amplification and Transmission
It is most convenient if the amplifier is sufficiently small to be carried by the patient, usually in a pouch placed on the chest or belt. After the amplifier, the transmitted signal is subject to much less artifact because it has been amplified. The amplifier should have minimal characteristics of a standard EEG amplifier. These are typically a low filter at 0.3 Hz and high filter at 60 or 70 Hz and sampling at 200 or 250 Hz. There is recent evidence that high frequencies in the intracerebral EEG (6, 7 and 8; see also Chapter 37) and low frequencies in newborns (9) may also be of interest. Optimum characteristics may include low frequencies (low filter at 0.1 Hz) and high frequencies (high filter at 600 Hz and sampling at 2000 Hz). Digital filters can be used when reviewing the EEG to eliminate unwanted low frequencies if they have been recorded. Recording with a high sampling rate has the disadvantage of increasing memory requirements and slowing down display. Twelve-bit digitizing is common and most likely to be sufficient, although 16-bit digitizing is also available and covers more than sufficiently the dynamic range of the intracerebral EEG.
Since the patient is monitored for extended periods, the cable system connecting the patient-worn amplifier to the computer or to a connector in the patient room must be light and comfortable, as well as very robust to withstand the stress of large seizures. The functions of the cable are to transmit the EEG signal and to provide power to the amplifier. Recent technology allows wireless transmission of the EEG, which gives much freedom of movement and added comfort to the patient. The drawback of wireless transmission is that the equipment carried by the patient must have a battery to power the amplifier as well as the wireless transmission. This significantly increases the weight of the patient-worn system and runs the risk of battery depletion, thus interrupting monitoring. Wireless transmission must therefore be considered for relatively short periods, with cable transmission used most of the time.
Video Recording
Digital video cameras are most commonly used. The usual color camera can be combined with an infrared illuminator and an automatic switching to black and white for low light conditions, thus providing an excellent quality image even in darkness. Allowing darkness increases patient comfort at night. The digital video signal is recorded continuously on the computer, synchronized with the EEG. The EEG amplifier and the video camera have independent clocks for sampling the EEG and for timing video frames. This makes the synchronization of the two signals a complex operation and it is important to verify that this synchronization is maintained.
Recording Computer, Display, and Network
The EEG and the video signals are transmitted to the computer, which may be at a distance from the patient room, either by dedicated cable or more and more commonly through a standard computer network (either the hospital network or a network dedicated to the LTM and EEG laboratories). Given the storage capacity of modern computer disks, there is no problem in storing several days of EEG recording, even sampled at high rates, and several days of video recording. Large monitors allow the easy display of 15 or 20 seconds of EEG on the screen. Characteristics of the EEG display, gain, time scale, and filters, can easily be changed. Since EEGs are usually recorded referentially, alternate montages can be viewed (bipolar, changed reference, average reference, etc.). Network facilities can be used to view the EEG from any computer connected to the computer on which the EEG is stored, either during the recording or after it is completed. This includes viewing from locations outside the hospital, such as at home, provided security barriers are respected.
Archiving the data is a challenge because it is not practical to archive all recorded data on permanent storage media such as DVDs, particularly the video, because of size. It is likely that complete archiving will become possible in a few years, but at present, the data that are to be archived must be selected by a person familiar with EEG interpretation to make sure all important information is retained.
Personnel and EEG Interpretation
Trained observers need to be near the patients to provide close clinical observation and interaction with the patient during
seizures, as well for ensuring patient safety. To assist with constantly observing patients on monitoring, patients can press an alarm button to report any auras or seizures they notice. Observation for seizures may be enhanced with automatic seizure detection systems (see below). It is also useful if a relative or companion can stay in the room with the patient and alert staff when seizures occur.
seizures, as well for ensuring patient safety. To assist with constantly observing patients on monitoring, patients can press an alarm button to report any auras or seizures they notice. Observation for seizures may be enhanced with automatic seizure detection systems (see below). It is also useful if a relative or companion can stay in the room with the patient and alert staff when seizures occur.
Experienced EEG technologists should be used so as to obtain the best information from an LTM session. They are critical to secure electrodes well and in the proper locations. They review the preliminary recorded data and seek to achieve suitable recording quality. In some centers they make a first review of the 24-hour session, marking the seizures and any section of interest. Only the marked sections are reviewed subsequently by the clinical neurophysiology physician. In other centers, the full recording is reviewed by the physician, but this is often considered too time consuming. Automatic seizure and spike detection may be helpful in selecting the sections to review (see below). In addition to selecting what data need review, it is necessary to decide what subset of the original recording needs to be permanently archived. (The full original recording is usually too large for storage, particularly the video.) The selection of what needs to be retained results from review and interpretation of the record. It typically consists of all seizures and some interictal spikes, as well as some sample of the EEG background.
Duration of Monitoring and Drug Withdrawal
The duration of monitoring depends on the purpose. In general, it is best to try to be generous with the duration of monitoring because it is frequent that unexpected events are discovered. Patients may report one seizure type but not be aware that they also have brief seizures of which they are not aware or nocturnal seizures nobody has witnessed. Some patients have more than one type of seizure, so a sample of only two to four seizures may be too few to use for major clinical decisions such as surgery. The clinical description reported by the patient or family is often incomplete or erroneous and it may require the recording of several seizures to fully document a seizure disorder that is different from what was expected. In the context of presurgical evaluation, it has been estimated that five seizures with the same region of onset and no contradictory information provide a reasonable sample to decide that the onset is indeed in that region (10).
Antiepileptic medications are frequently reduced or discontinued during LTM in order to allow seizures to occur more frequently. In the context of presurgical evaluation, the question of whether seizures occurring after medication withdrawal are similar to habitual seizures has been raised. Symptomatology and the EEG pattern at onset are similar after medication withdrawal (11, 12, 13 and 14). There is no evidence that seizures spread more rapidly from one hemisphere to the other after drug withdrawal. Seizures are more often secondarily generalized after drug reduction (15,16), so monitoring unit staff should be prepared to respond in case of repeated generalized seizures.
It is also often said that the reduction in antiepileptic medication results in increased spiking activity. Careful studies of the timing of drug withdrawal, seizure occurrence, and changes in spiking (17,18) have shown that spiking does not increase following drug withdrawal per se. Spiking does increase following seizures. When drugs are reduced, seizures occur and spiking increases as a result of increased seizures. It may therefore appear that drug reduction results in increased spiking, but this is an indirect effect mediated by seizures. In the absence of seizures, spiking does not change. These observations were confirmed in a recent study using more sophisticated analysis tools (19). This study also demonstrated that spiking could even decrease as a result of drug withdrawal in the absence of seizures.
Considerations for Children or Infants and for Adults
LTM can be performed at all ages including during the newborn period (20). In newborns and young infants, a subset of the standard 10/20 electrodes can be used although one has to be careful because seizures can be very focal. Collodion should not be used in young children, as their skin is very delicate. As seizures and interictal activity are more frequent in young patients than in adults, the duration of monitoring is usually shorter, often limited to 1 or 2 days. In children, a full syndromic definition often requires that different seizure patterns be recorded, as well as the interictal activity (3). In adults but more particularly for children, it is important to have a parent in the room during monitoring to help identify the events being investigated.
Automatic Seizure and Spike Detection
The size of computer disks is such that it is simple to perform a continuous 32-channel, 7-day recording on a standard computer, storing EEG and video. A computer-based recording allows a flexible review procedure, with random access to any part of the recording and the availability of numerous methods of data manipulation and analysis. In most 24-hour recordings, 95% to 99% of the recording offers little useful information in the evaluation of an epileptic disorder. Automatic detection methods, despite their imperfections, can facilitate the marking and thus the review of valuable information.
Automatic Seizure Detection
During LTM, patients are usually asked to press a button when they feel a seizure coming. If patients could always feel their seizure coming, there would be no need for automatic seizure detection. It is frequent, however, that patients are not aware of their seizures, and observers are not always present or able to recognize a seizure. Furthermore, some seizures have very minimal clinical signs—and sometimes no visible signs at all. Finding all seizures may require the review of days and days of EEG, a process that is long and tedious—and not always effective in as much as short seizures can be missed, particularly if there are many channels as in intracerebral recordings. Automatic seizure detection can be helpful in this context, since it provides a means for marking the EEG sections that are likely to include seizure patterns. For this purpose, the detection can take place at any time during the seizure (not necessarily early) because the EEG will be reviewed a posteriori.
Seizure detection could also be used in real time, to warn the patient or observer that a seizure has just started. In this case, the detection must occur early to allow the patient to take protective measures (if he or she is still able to do so), to assist an observer in providing protective measures in a timely fashion, and to facilitate the observer’s attempts to question the patient so as to improve our understanding of clinical signs (e.g., can the patient follow commands, talk, remember?). Such an early seizure detection system can be used during monitoring in the clinic (21,22), but could also be incorporated in an implantable device that would provide a warning (e.g., auditory signal) to the patient during normal everyday activities.
Seizure prediction is an area of active investigation but, unlike seizure detection and the warning provided by the onset of the seizure, it is an area that has proven difficult and without clear results. Despite initially promising results, it is not yet clear if it is possible to predict seizures (23; see also Chapter 30).
Detection Methods
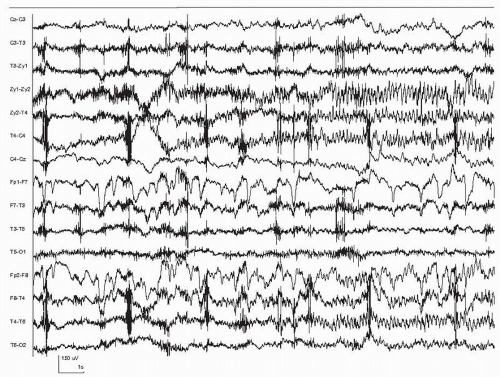
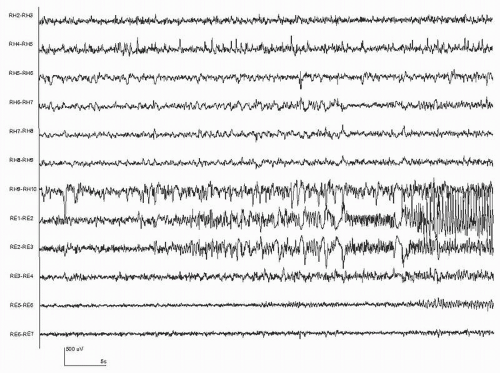
Our current understanding is that seizures consist of paroxysmal rhythmic activity generated by a large number of synchronized neurons. This activity usually evolves in its spatial distribution, frequency, and amplitude. The patterns that are actually recorded depend not only on the discharge itself, but also on the respective position of the electrodes and the generator. In some cases, no seizure discharge may be visible because the electrodes are too far from the generator. Similarly, the seizure discharge may appear only as a mild modification to the ongoing EEG. It is therefore important to distinguish the seizure itself from its manifestations (i.e., from the vantage point of the recording electrodes). Since seizure detection is performed from analyzing the EEG, it is only the seizures that have a clear manifestation in the EEG that can be detected. Figures 35.1 and 35.2 show examples of seizures recorded from the scalp, and Figure 35.3 shows a seizure recorded from intracerebral electrodes. In Figures 35.1 and 35.3, the specific moment of onset is not easily identified. In Figure 35.2, the onset is clear, consisting of a few seconds of rhythmic discharge followed by irregular slow waves.
There is no formal definition of what a seizure consist of in the EEG. Qualitative definitions usually include the terms “paroxysmal, rhythmic, evolving”— but an unambiguous definition has not been formulated. Automatic detection methods have therefore relied on trying to encode these qualitative characteristics into algorithms, and improving the algorithms by trial and error in an effort to increase detection sensitivity and decrease the false detection rate. The first general-purpose seizure detection method validated on a large data sample, characterized each
2-second epoch by measures of frequency, rhythmicity, and amplitude change, and compared these measures to a constantly updated background (24,25). A set of empirical rules combined the findings in multiple epochs and multiple channels to reach a decision on detection. The concept of a “constantly updated background” plays an important role in the analysis of long-term data, since EEG is nonstationary, that is, the background content changes considerably due to factors such as states of vigilance. It is also important that the results not be dependent on the selection of a particular background epoch, a dependence that would introduce arbitrariness into the process. More recent methods that have followed a similar empirical approach include those of Grewal and Gotman (26), and Saab and Gotman (27).
2-second epoch by measures of frequency, rhythmicity, and amplitude change, and compared these measures to a constantly updated background (24,25). A set of empirical rules combined the findings in multiple epochs and multiple channels to reach a decision on detection. The concept of a “constantly updated background” plays an important role in the analysis of long-term data, since EEG is nonstationary, that is, the background content changes considerably due to factors such as states of vigilance. It is also important that the results not be dependent on the selection of a particular background epoch, a dependence that would introduce arbitrariness into the process. More recent methods that have followed a similar empirical approach include those of Grewal and Gotman (26), and Saab and Gotman (27).
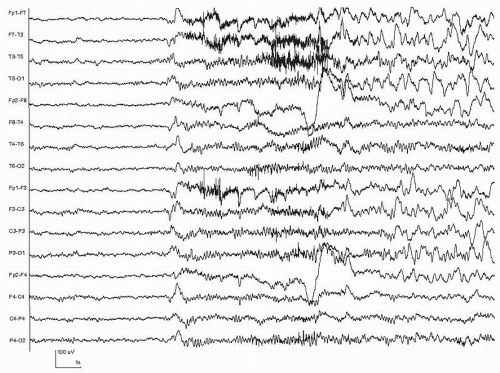 Figure 35.2 Scalp-recorded seizure with a generalized and abrupt onset. A few seconds of rhythmic theta frequency activity are followed by irregular slow waves in the delta range. |
Artificial neural networks (ANNs) are an interesting approach to seizure detection (28, 29 and 30). The ANN is presented with a large number of training examples that comprise both seizure and nonseizure data, and gradually learns to differentiate seizures from nonseizure EEG. A set of features characterizing the EEG needs to be specified in advance (e.g., amplitude, rhythmicity, dominant frequency, etc.), but it is not necessary to explicitly describe seizure patterns. The ANN automatically determines which combinations of features are characteristic of seizures by adapting its internal structure to reflect the training data. The performance of ANN methods depends on an appropriate set of features and on exposure to a sufficiently large training set containing a variety of seizure patterns.
An extension to the above concept of context is to incorporate a particular patient’s seizure characteristics in the context. In this case, the method is aimed at detecting seizures in that particular patient, and only seizures that look like the sample seizures (that have been incorporated in the context) are detected. The method can then be labeled “patient-specific”— or more correctly, “seizure-specific”—since a patient can have several types of seizure. Better detection performance can thus be obtained if it is acceptable to detect only a specific seizure pattern in a given patient (21,22,31,32).
Seizure patterns are quite different in the newborn EEG than in older children and adults: the discharges are often much slower and sometimes very focal (limited to one electrode). For these reasons, methods specific to the newborn have been developed, with the particular aim of detecting the very slow discharges often present during newborn seizures (33, 34 and 35).
Validation of Detection Methods
A method must be tested on a data set independent from that on which it was developed. The testing data must be representative of the context in which the method will be used: they should include a large variety of seizures, and a much larger amount of nonseizure data than of seizure data (in order to ensure that all types of interictal data are well represented— quiet and active wakefulness, different sleep stages, different types of artifacts common during LTM). The best way to avoid
selection bias is probably to select all data between two given dates. An important issue in validating a method is the definition of a seizure itself. Whereas there is no ambiguity with a clear seizure lasting 30 seconds or 1 minute, significant questions arise for short events: What is the minimum duration of a seizure? Should a seizure having clinical manifestations, but no change in the EEG, be counted as an event to detect? What if the EEG changes are very minor, such as a brief flattening or a short burst of slow waves? When presenting validation results, these issues should be addressed.
selection bias is probably to select all data between two given dates. An important issue in validating a method is the definition of a seizure itself. Whereas there is no ambiguity with a clear seizure lasting 30 seconds or 1 minute, significant questions arise for short events: What is the minimum duration of a seizure? Should a seizure having clinical manifestations, but no change in the EEG, be counted as an event to detect? What if the EEG changes are very minor, such as a brief flattening or a short burst of slow waves? When presenting validation results, these issues should be addressed.
Methods that were evaluated on extensive data sets (hundreds of hours, tens of patients) report detection sensitivities from 75% to 90%. Sensitivity is often lower in newborns than in adults, as some newborn seizure patterns are very subtle. The false detection rate, the most common measure of failure, is in the range of 0.3 to 1 per hour in recent studies. Few studies report results with intracerebral electrodes, but these recordings have important distinguishing characteristics: scalp artifacts (electromyogram [EMG], movement, eye blinks) are absent, thus reducing false detections; the dynamic range of spontaneous fluctuations in background is much larger in intracerebral than in scalp EEG—thus resulting in more false detections; the intracerebral EEG often includes many paroxysmal bursts of uncertain significance, which can also contribute to false detections; and seizure patterns are often more prominent in intracerebral EEG, thus resulting in better sensitivity (26).
Automatic Spike Detection
Many epileptic patients present with interictal abnormalities in their EEG. These consist of abnormalities of the background, which will not be discussed here, and paroxysmal events such as spikes, polyspikes, sharp waves, and bursts of spike and wave. These paroxysmal events occur most often unpredictably and sometimes rarely. They play an important role in defining some epileptic syndromes and they contribute to localizing the source of seizure discharges. Although used primarily for these purposes, it is important to remember that they, by themselves, disturb normal brain function (36,37). Automatic spike detection methods started to be developed when computer analysis of the EEG was in its infancy, in the 1960s. Despite this long history and despite the tremendous increase in the power of computers over this period, automatic detection today is far from perfect. There are probably two reasons for this: the first is that spikes are not a well-defined pattern, as illustrated by the low
inter-rater agreement demonstrated in the study of Wilson et al. (38); it is an almost impossible task to detect a pattern for which there is so little agreement. The second reason may be that, despite its apparent simplicity, spike detection is quite a difficult task that the human brain performs rapidly but that takes into account a complex analysis of temporal and spatial features as well as context, an analysis that is not easy to reproduce in a computer program.
inter-rater agreement demonstrated in the study of Wilson et al. (38); it is an almost impossible task to detect a pattern for which there is so little agreement. The second reason may be that, despite its apparent simplicity, spike detection is quite a difficult task that the human brain performs rapidly but that takes into account a complex analysis of temporal and spatial features as well as context, an analysis that is not easy to reproduce in a computer program.
During routine EEG examinations or short recordings, automatic spike detection is of little use for an expert because it is easy to find all the spikes visually. It is sometimes said, however, that nonexperts overinterpret the EEG, defining as epileptiform transients that are artifacts or normal variants (39). This can result in overtreatment of patients. In such a context a reliable automatic detection method would be useful. Generally, automatic detection becomes valuable mostly when analyzing long recordings, particularly during long-term epilepsy monitoring, when hours or days of recording have to be examined. It is also in this context that automatic detection is most difficult because the recording includes multiple transients that are difficult to differentiate from epileptiform transients: eye blinks, movement and muscle artifacts, electrode artifacts, sleep transients (vertex waves, spindles, K complexes).
An excellent review of spike detection methods for scalp EEG has recently been published by Halford (40) and we refer the reader to this comprehensive review. No spike detection method has addressed specifically the problem of detecting spikes in the intracerebral EEG. Although in appearance it is an easier problem than detection in the scalp EEG because of the absence of most of the artifacts, it remains a difficult problem because the issue mentioned above regarding the definition of spikes is worse in the intracerebral EEG. Some channels show almost continuous sharp activity and it is very difficult to identify isolated transients. The morphology of sharp transients is also highly variable. In other words, the problem is even more ill-defined than for scalp spikes.
LONG-TERM ICU MONITORING
ICU EEG monitoring is used for several clinical purposes. In coma EEG can search for possible presence of seizures, some of which are nonconvulsive. For patients at risk for deterioration, EEG can monitor continuously for adverse signs. For patients in a deliberate burst suppression state, EEG can monitor that the induced coma is sufficiently deep.
A primary goal of ICU EEG monitoring is identification of acute adverse events early enough to intervene to prevent or ameliorate long-term adverse clinical sequellae. Examples of acute events in neurologic critical care patients include subarachnoid hemorrhage (SAH), intracranial bleeding, vascular thrombosis or embolus, convulsive or nonconvulsive seizures, cerebral edema, or vasospasm. Some are beyond meaningful intervention. Many are amenable to various medical or surgical therapies.
Typical Indications for ICU EEG Monitoring
Monitoring can identify subtle or nonconvulsive seizures. Seizures can manifest in various ways and circumstances. EEG can determine whether a critically ill patient is having seizures, including nonconvulsive seizures, that cause a decreased level of consciousness (41,42). Nonconvulsive seizure EEG patterns may be frequent to constant epileptic spiking, or more discrete cycles of ictal discharges separated by interictal attenuation and slowing. Spike morphology can be the traditional single spike or sharp wave events, or more organized trains of spike wave seen in focal or generalized epilepsy (43, 44, 45, 46, 47, 48, 49, 50, 51 and 52). EEG monitoring of critically ill patients is indicated for patients with decreased level of consciousness not otherwise explained.
ICU EEG monitoring can separate movements from seizures. For example, pseudoperiodic lateralized epileptiform discharges (PLEDs) can be seen at the site of focal lesions, such as ischemic stroke (53,54). They may be accompanied by myoclonic jerks, usually seen as temporally separate rather than synchronous with the PLED discharges. EEG recordings with EMG channels accompanying the myoclonic jerks can demonstrate the lack of temporal relationship of jerks to EEG discharges, evidence that observed movements are myoclonic rather than epileptic. Some medications, for example, tacrolimus, are associated with tics or other repetitive movements for which EEG with an EMG channel or video-EEG ICU monitoring can help determine whether movements are epileptic.
Monitoring can grade the degree of encephalopathy. Slowing in routine EEGs can be either continuous or intermittent. A fixed structural deficit or anoxic injury tends to produce continuous slowing. A metabolic encephalopathy tends to produce waxing and waning of background, that is, intermittent slowing. For both the continuous and intermittent slowing, the degree of EEG slow activity corresponds in a general way with the degree of impairment. For example, alpha coma from hypoxic injury is characterized by monotonously continuous frontocentral alpha activity (55, 56, 57, 58, 59, 60 and 61) that is nonreactive, that is, does not change when the patient is stimulated. EEG monitoring can evaluate the EEG not only for reactivity to stimulation and moment to moment monotony, but also for the spontaneous variability that occurs more normally over many minutes to hours. Presence of such variability is a favorable sign, and absence is unfavorable. Shorter routine EEGs may miss these longer duration fluctuations in EEG content because the sample is too short. ICU EEG monitoring can identify and measure the variability in long-term EEGs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree