Chapter 44 Principles of Pain Management
Anatomy and Physiology of the Pain Pathways
Axons of DRG neurons send the primary nociceptive afferents through the dorsal roots to the most superficial layers of the dorsal horns (Rexed laminae I and II) and to some of the deep laminae (Rexed V). The Aδ fibers conveying input from HTMs and MTs terminate primarily in laminae I and V; C fibers mainly terminate in lamina II. Neurotransmitters related to pain conduction include excitatory amino acids and neuropeptides, particularly substance P (Geracioti et al., 2006). The second-order neurons in the dorsal horn include cells that respond only to noxious stimuli (nociceptive specific neurons) and others (wide dynamic range [WDR] neurons) that respond to both nociceptive and non-nociceptive sensory stimuli.
Central Modulation of Nociception
Descending inhibitory systems appear to have three functionally interrelated neurotransmitter mechanisms: the opioid, the noradrenergic, and the serotonergic systems. Opioid precursors and their respective peptides (β-endorphin, methionine [met]-enkephalin, leucine [leu-] enkephalin, and dynorphin) are present in the amygdala, hypothalamus, PAG, raphe magnus, and the dorsal horn. Noradrenergic neurons project from the locus caeruleus and other noradrenergic cell groups in the medulla and pons. These projections are found in the dorsolateral funiculus. Stimulation of these areas produces analgesia, as does the administration (direct or intrathecal) of α2-receptor agonists such as clonidine (Khodayar et al., 2006). Many serotonergic neurons are found in the raphe magnus. These neurons send projections to the spinal cord via the dorsolateral funiculus. Administration of serotonin to the spinal cord produces analgesia, and pharmacological blockade or lesion of the raphe magnus can reduce the effects of morphine. The antinociceptive effects of antidepressants such as tricyclics and newer serotonin-norepinephrine reuptake inhibitors such as duloxetine and milnacipran are believed to reduce pain by increasing serotonin and norepinephrine concentrations in descending inhibitory pain pathways.
Opioid Receptors
The δ receptor has similar central and peripheral distribution as the µ receptors. Studies have shown that δ-opioid agonists can provide relief of inflammatory pain and malignant bone pain. Meanwhile, peripherally restricted κ-opioid agonists have been developed to target κ-opioid receptors located on visceral and somatic afferent nerves for relief of inflammatory, visceral, and neuropathic chronic pain. The potential analgesic effects, combined with a possible lower abuse rate and fewer side effects than µ-receptor agonists, makes δ- and peripherally restricted κ-opioid receptor agonists promising targets for treating pain (Vanderah, 2010).
Central Sensitization
Central sensitization plays a major role in the development of neuropathic pain syndromes. First, postsynaptic depolarization in the spinal cord in response to afferent stimulation can induce removal of magnesium blockade in N-methyl-d-aspartate (NMDA) receptors such that glutamate now induces a depolarization upon receptor binding. This process is short lasting and is called wind up (Katz and Rothenberg, 2005). It is responsible for the temporal summation of inputs. The second set of changes is related to phosphorylation of the NMDA receptor, which is a key process for longer-lasting changes in the excitability of the dorsal horn neurons that produce central sensitization. This posttranslational modification of the NMDA receptors results in dramatic changes in excitability due to removal of the voltage-dependent magnesium block in the absence of cell depolarization and also to changes in channel kinetics, such as channel opening time. NMDA receptor activation allows calcium influx into the cell, which further augments signal transduction within the dorsal horn neurons by activating a number of intracellular signal transduction kinases. As a result, relatively brief C-fiber inputs initiate very rapid changes in membrane excitability. This manifests both as a progressive increase in excitability during the course of the stimulus (wind up) and as post-stimulus changes that may last for several hours (central sensitization).
Clinically, the real meaning of peripheral and central sensitization is the enhanced and prolonged pain perception to minor stimulations, or sometimes without peripheral stimulation. Once peripheral and central sensitizations are involved, the pain is usually more difficult to treat. It is now believed that peripheral and central sensitization may be involved in a wide variety of chronic pain conditions such as reflex sympathetic dystrophy, tension headache, carpal tunnel syndrome, pain after spinal cord injury (Carlton et al., 2009), and even in pain conditions previously thought to be mainly nociceptive in nature such as fibromyalgia, epicondylalgia, and osteoarthritis (Gwilym et al., 2009). The challenge to the clinician is that when trying to make a diagnosis for a pain patient, we should not only try to localize the pain source—as most clinicians always do—we should also factor in the role of peripheral and central sensitization and what the best treatment strategy will be in each case.
Common Pain Syndromes
Trigeminal Neuralgia
The diagnosis of TN is based primarily on a history of characteristic paroxysmal pain attacks. The White and Sweet criteria are still commonly used worldwide (Box 44.1). In the majority of TN patients, the clinical examination, imaging studies, and laboratory tests are unremarkable (classic TN). In a smaller group, TN is secondary to other disease processes affecting the trigeminal system (symptomatic TN). Because a significant percentage of patients have symptomatic TN resulting from other disease processes, diagnostic MRI studies should be part of the initial evaluation of any patient with TN symptoms. Special attention should be paid to MS plaques, tumor, and subtle vascular anomalies that may be the source of root compression. Recent studies found that high-resolution three-dimensional (3D) MRI reconstruction and magnetic resonance cisternography may provide alternative tools to better identify the presence of neurovascular compression and even measure the volume of neurovascular compression at the cerebellopontine angle and predict the prognosis after initial treatment (Tanaka et al., 2009).
Carbamazepine is the first choice for treatment of TN; both controlled and uncontrolled studies confirm its clinical efficacy. Carbamazepine monotherapy provides initial symptom control in as many as 80% of TN patients. Of those initially responding to the drug, approximately 75% will continue to have long-term control of pain attacks. Controlled studies demonstrate that baclofen and lamotrigine are superior to placebo for treatments of TN. In the experience of many clinicians, baclofen is just as effective as carbamazepine and often better tolerated. A recent study found that oxcarbazepine may be effective for those who were unresponsive to the treatment of carbamazepine (Gomez-Arguelles et al., 2008). Pregabalin may also be potentially effective. If a patient is not satisfied with single medication therapy, adding another oral medication may offer additional benefits. Intravenous (IV) lidocaine or phenytoin could be effective for some severe refractory cases of TN. However, these treatments carry additional risks and require close cardiovascular monitoring. Opioid analgesics have not been proven effective for TN and should be avoided.
Posterior fossa exploration and microvascular decompression (MVD) is assumed to directly treat the cause of TN. However, this is a complex and invasive therapy with a possibility of death. With the availability of other less-invasive procedures, MVD is infrequently used and is only reserved for younger and healthier patients. Several studies demonstrated trigeminal radiofrequency rhizotomy successfully controls symptoms in over 85% of TN cases. The technique is minimally invasive. To heat the gasserian ganglion, a radiofrequency needle is inserted through the foramen ovale under the guidance of fluoroscopy. The procedure can be finished in less than 30 minutes in experienced hands. A few patients experience sensory loss and dysesthesia (analgesia dolorosa) in the distribution of the damaged trigeminal fibers with this procedure. Stereotactic radiosurgery (SRS) employs computerized stereotaxic methods to concentrate ionizing radiation on the trigeminal root entry zone. Several studies have demonstrated the high clinical efficacy and relative safety of this new technique. It is currently recommended as a first-line noninvasive surgical technique in many pain centers, especially for frail or elderly patients (Zahra et al., 2009).
Cervicogenic Headache
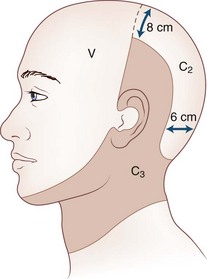
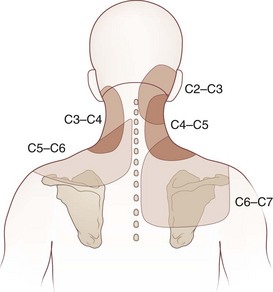
Cervicogenic headache refers to head pain originating from pathology in the neck. It is believed that pain from the C2-C3 nerve dermatome can radiate to the head and face (Fig. 44.1). An earlier study found that pain from the C2-C3 and C3-C4 cervical facet joints also can radiate to the occipital area (Fig. 44.2). The term cervicogenic headache was first introduced by Sjaastad and colleagues in 1983. However, the concept of cervicogenic headache is controversial and not well accepted by the majority of neurologists. The International Headache Society (2004) published its first diagnostic criteria in 1998 and revised it 2004. Patients with cervicogenic headache often have histories of head and neck trauma. Pain may be unilateral or bilateral. Pain is frequently localized to the occipital area, but it may also be referred to the frontal, temporal, or orbital regions. Headaches may be triggered by neck movement or sustained neck postures. This headache is constant with episodic throbbing attacks like a migraine. Patients may have other symptoms mimicking a migraine, such as nausea, vomiting, photophobia, phonophobia, and blurred vision. Owing to significant overlap of the symptoms of cervicogenic headache and migraine without aura, cervicogenic headache is often misdiagnosed as migraine. Clinicians should always consider cervicogenic headache in the differential diagnoses when evaluating a headache patient. History of head/neck injury and detailed examination of the occipital and upper cervical area should be part of the evaluation for headache. Patients with cervicogenic headache may have tenderness over the greater or lesser occipital nerve, cervical facet joints, and muscles in the upper or middle cervical region. Cervicogenic headache does not respond well to migraine medications. Treatment should be focused on removal of the pain source from the occipital-cervical junction. Initial therapy is directed to physical therapy modalities and NSAIDs. Interventional treatment such as greater occipital nerve block, cervical facet joint block, deep cervical plexus block, and botulinum toxin injections may provide effective pain relief (Zhou et al., 2010).
Complex Regional Pain Syndrome
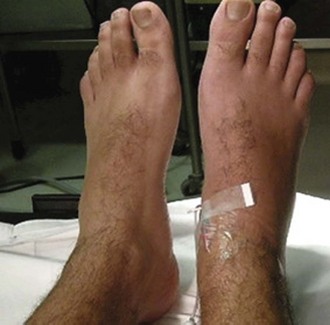
The mean age of CRPS patients ranges from 36 to 46 years, with women predominating (60%-81%). It is caused typically by an injury such as a fracture (16%-46%), strain or sprain (10%-29%), post surgery (3%-24%), and contusion or crush injury (8%-18%). Clinical features of CRPS often include pain, edema, autonomic dysfunction such as change in temperature or color in the involved limbs, motor dysfunction, and psychological abnormalities such as depression (Fig. 44.3). Schwartzman and Maleki reported the pattern of spreading of CRPS in three stages. In the early stage, CRPS often involves only one limb with pain, minor edema, and increased skin temperature. CRPS may spread from one limb to the others. In the later stage, CRPS could involve the full body and the four extremities with severe pain, edema, cold and cyanotic limbs, joint contracture, and atrophy of muscles and bones.
Tricyclic antidepressants, antiepileptics, and narcotics such as methadone are commonly used empirically for CRPS, even though clinical controlled studies have not proven their efficacy. A recent review article summarized the evidence derived from randomized controlled trials pertaining to the treatment of CRPS. The review reported clinical improvement with dimethyl sulfoxide, steroids, epidural clonidine, and intrathecal baclofen. Only bisphosphonates appear to offer clear benefits for patients with CRPS (Tran de et al., 2010). NMDA receptor modulation is a major interest of current research. It has been reported that subanesthetic infusions of ketamine might offer a promising therapeutic option in the treatment of appropriately selected patients with intractable CRPS (Schwartzman et al., 2009). A recent preliminary study reported that IV immunoglobulin treatment could potentially decrease pain in CRPS patients (Goebel et al., 2010). However, more studies are needed to further establish the safety and efficacy of these novel approaches.
Minimally invasive techniques have been used extensively for the treatment of CRPS. Techniques include sympathetic block, intravenous regional block (IVRB), somatic nerve block, epidural drug administration, intrathecal drug delivery, and neurostimulation. Stellate ganglion blocks in early-stage CRPS may significantly decrease pain and hasten clinical recovery. It may also prevent the recurrence of CRPS after reoperation of the affected extremity. In a double-blind study, IVRB with bretylium provided significantly longer analgesia than lidocaine. Good pain relief is reported with the use of epidural delivery of clonidine and ketamine and also with intrathecal baclofen and morphine. An early study with 2-year follow-up reported that spinal cord stimulation (SCS) results in a long-term pain reduction and improvement in health-related quality of life. However, a more recent randomized study with 5-year follow-up found no extra benefit in terms of pain relief for those with a combination of SCS and physical therapy, compared to those with physical therapy alone (Kemler et al., 2008). The author shares the same experience and opinion with the cited report. It seems that most RSD patients feel better immediately after the SCS implantation. However, the SCS itself may have difficulty stopping the spread of RSD, and once RSD spreads out of the area initially covered by the SCS, the pain is no longer “under control.”
Post-Stroke Pain Syndrome
Treatment of central post-stroke pain remains a challenge. Tricyclic antidepressants are still a choice of treatment. Gabapentin and lamotrigine have been used to treat central post-stroke pain syndrome in open-labeled studies. Selective posterior rhizotomy has been reported to decrease painful spasticity in the lower limbs of hemiplegic patients after a stroke. It has been reported that chronic motor cortex stimulation therapy provides pain relief for some post-stroke patients (Brown and Pilitsis, 2006). Stereotactic radiosurgery of the pituitary and deep brain stimulation (DBS) have been used to treat PSP syndrome with some success (Pickering et al., 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree