Figure 7.1 Brain in Cerebral Malaria (CM)
Clinical features
The first symptoms of cerebral malaria in adults include fever, headache, myalgia, malaise followed by progressive drowsiness, confusion, delirium, stupor and coma. These symptoms usually develop over 1-3 days but may occur in <24 hours. The onset can be relatively sudden with the patient presenting with a febrile illness over hours followed by a generalised seizure and or coma. This is a more common presentation in children and in non immune hosts. Seizures occur in >50% of children and about 20% of adults, either at onset or throughout the course of the illness. In adults the main neurological findings are those of an acute encephalopathy with symmetrical upper motor neurone signs. These include altered level of consciousness, divergent gaze, bruxism (teeth grinding), hypertonia and extensor plantar reflexes. The duration of coma after starting treatment is on average 1-3 days but may persist for longer in adults. Systemic complications include anaemia, acidosis, renal failure, respiratory distress syndrome and secondary bacterial sepsis.
A characteristic malaria retinopathy has recently been described in Malawi occurring in children and also in adults with cerebral malaria (Fig. 7.2). This retinopathy is characterized by areas of retinal whitening best seen immediately around the macula/fovea but sparing it, coupled with white or orange discolouration of some retinal vessels and capillaries. These features are now considered to be specific for cerebral malaria. They are best seen with the direct ophthalmoscope provided that the pupils have been dilated and the examiner is already familiar with them. The more classical fundoscopy findings in cerebral malaria include retinal haemorrhages (<10% of adults) and papilloedema (<1% of adults). Characteristic white centered retinal haemorrhages are found in most children and papilloedema in 10%. These however are not specific for cerebral malaria.
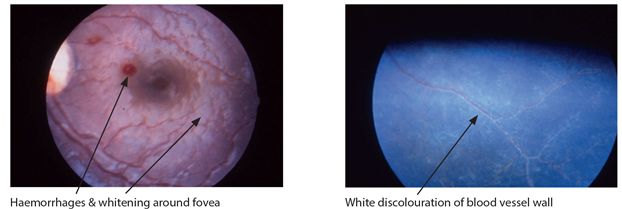
Figure 7.2 Retina in CM. Photographs courtesy of Susan Lewallen Ophthalmology Dept, KCMC
Differential diagnosis
The high prevalence of asymptomatic parasitaemia may make the diagnosis less certain and therefore other causes of encephalopathy need to be excluded. Infectious causes include acute bacterial meningitis (ABM), partially treated ABM, viral meningoencephalitis including rabies, semi acute presentations of the main HIV related CNS infections including cryptococcal and tuberculous meningitis and toxoplasmosis. Non-infectious causes include metabolic abnormalities, intoxication, epilepsy, stroke and other causes.
Diagnosis of malaria
The diagnosis of cerebral malaria is supported by laboratory evidence of infection. This is usually done by the demonstration of malaria parasites on a peripheral blood slide. A blood slide, usually a thick film is taken first on admission, after the first 24 hours and again at 48 hours. A negative blood slide on admission needs to be repeated if cerebral malaria is still suspected.
Newer methods of diagnosis include rapid diagnostic tests (RDT) which detect parasitic enzymes or antigen in whole blood. Most are sensitive and specific for P. falciparum and are very helpful particularly where skilled microscopy is unavailable. RDTs based on antibody detection are of less value clinically as they don’t distinguish between old or recent infection and are non specific for falciparum.
A full blood count is frequently normal but may show anaemia especially in children. The presence of leucocytosis and thrombocytopenia usually indicates severe systemic malaria or coexistent sepsis. Hypoglycaemia is common in severe malaria particularly in children and blood glucose should always be regularly checked in cerebral malaria. Other investigations include renal, liver function, coagulation screen, arterial and blood gases. Urine in malaria may rarely be dark or black in colour and an analysis shows red blood cell casts. CSF examination is clear in colour with no increase in cells but may show a slightly elevated opening pressure with a mild elevation in protein. Neuroimaging is typically normal.
Key points
- cerebral malaria is a major cause of death in children <5 yrs
- clinical features include headache, fever, myalgia, altered consciousness & seizures
- symptoms typically progress over 1-3 days
- diagnosis is supported by evidence of malaria parasites in blood
- other causes of encephalopathies need to be excluded
Treatment of cerebral malaria
The treatment of a patient with cerebral malaria is based on specific drug treatment and the early recognition and management of complications. Management of complications includes urgent measures to treat hypoxia, hypoglycaemia, seizures, hypovolaemia, anaemia and acidosis. Because most deaths in cerebral malaria occur within 24 hours of onset, patients should if possible be admitted to a high dependency care area or an intensive care unit and have emergency management. Blood sugar should be checked every 4-6 hours particularly in children because of recurrent hypoglycaemia. Fluids need to be restricted in the first 48 hours, if raised intracranial pressure is suspected. Complicated cases may need intubation, ventilation, exchange transfusion and dialysis if available. A summary of the steps in emergency management of complications is outlined below.
Management of complications of cerebral malaria
- maintain airway with oxygen if hypoxaemic or in respiratory distress
- if high fever present reduce temperature with paracetamol
- correct hypovolaemia in children with NS, infusion @ 2-3 ml/kg/hr↓
- treat hypoglycaemia with iv bolus one ml/kg of 50% dextrose
- give 5-10% dextrose infusion to all cerebral malaria patients to prevent hypoglycaemia
- control seizures using either diazepam or lorazepam or phenytoin/phenobarbitone
- offer blood transfusion if the haematocrit is <15% in children or <20% in adults
- give fresh whole blood, frozen plasma and vitamin K for spontaneous bleeding
- give first-line antibiotics for possible pyogenic meningitis and sepsis until excluded
- ventilate adults with respiratory distress syndrome and refer for dialysis if in renal failure
Specific drug treatment
The specific drug treatment of cerebral malaria includes the artemisinin compounds and quinine (Table 7.1). Recent studies in severe malaria show that parenteral artemisinin compounds are superior to quinine; they are easier to administer, better tolerated with no major side effects and have a better outcome. Intravenous artesunate was shown to be more potent than quinine (35% greater reduction in mortality rate) in treating cerebral malaria in adults in Vietnam. It is also more potent than artemether, possibly because of a more rapid effect. Parenteral artesunate is now the drug of first choice in the treatment of cerebral malaria in Africa.
Table 7.1 Drug treatment of cerebral malaria
| Drug | route | Dose loading | Dose maintenance | Duration |
| artesunate | iv | 2.4 mg/kg | 2.4 mg/kg/ @ 12 and 24 hours & then daily | 7 days |
| or | ||||
| artemether | im | 3.2 mg/kg | 1.6 mg/kg/daily | 5 days |
| or | ||||
| quinine dihydrochloride | iv | 20 mg/kg (max 1400 mg) over 4 hours in 5% dextrose or dextrose/saline | 10 mg/kg/8 hourly* | 5 days |
* infused over four hours in adults & changed to oral as soon as the patient can swallow
Table 7.2 Blantyre coma scale*
| To obtain coma score add the scores from each section** | |
| Best motor response Localizes painful stimulus Withdraws limb from painful stimulus No or inappropriate response | 2 1 0 |
| Best verbal response Cries or speaks appropriately with painful or verbal stimuli Moans or abnormal cry to painful stimulus No vocal response to painful stimulus | 2 1 0 |
| Eye Movements Watches or follows e.g. mother’s face Fails to watch or follow | 1 0 |
* for use in children
** Score ≤2 & a positive blood slide suggests cerebral malaria
Meanwhile quinine is still being used for the treatment of cerebral malaria in many countries in Africa and its use should never be delayed if artemisinins are unavailable. The main side effects of quinine are cinchonism (tinnitus, deafness, dizziness, nausea and vomiting), cardiac depression, hypotension, hypoglycaemia, blindness and very rarely blackwater fever. Quinine should also be used cautiously in patients with heart disease and in the elderly. In adults a loading dose of 20 mg/kg is given iv over 2-4 hours and then continued by iv infusion at 10 mg/kg/8 hourly for 5 days or until able to take orally. After 3 days of iv treatment the dose can be reduced from 8 to 12 hourly. Intramuscular quinine administered according to instructions can be used if iv route is not possible.
Prognosis in Cerebral Malaria (CM)
The mortality rate in quinine and artemisinin treated children with cerebral malaria in Africa is 15-20%. Mortality rates are lower <10%, in both artemisinin and quinine treated adult patients. However mortality rates in pregnancy are up to 50%. Risk factors for death in cerebral malaria in adults include anaemia, seizures, respiratory distress syndrome and renal failure. Prolonged and deep coma, elevated intracranial pressure and hypoglycaemia are risk factors in children. While the majority of patients make a full recovery from treated cerebral malaria, permanent neurological deficits still occur in >20% of children and <5% adults. Deficits including psychoses, ataxias are usually transient and clear within weeks or months. Gross neurological deficits occur in about 10% of children, these include, hemiparesis, quadriparesis, cerebellar ataxia, and severe brain damage. Neurocognitive and behavioural dysfunction are also more common occurring in >20% and epilepsy occurs in about 10%. Hemiparesis is the main deficit in adults.
Key points
- parenteral artemisinin is superior to quinine in treatment of cerebral malaria
- mortality in treated children is 15-20% and <10% in adults
- morbidity occurs in >20% of children and <5% adults
- morbidity (children), neurological & cognitive deficits, behavioural abnormalities & epilepsy
- hemiparesis is most frequent complication in adults
TOXOPLASMOSIS
Introduction
Toxoplasmosis is caused by infection with Toxoplasma gondii. T. gondii is an intracellular protozoan parasite whose definitive host is the cat. Humans and other animals may become infected accidentally from infected cats via ingestion of food or water contaminated with cat faeces. Transmission in Africa is most probably from ingestion of undercooked meat of infected animals. Human infection occurs mostly during childhood and antibody seroprevalence rates are a measure of latent infection. Worldwide seroprevalence rates vary from 20% to 75% and a similar pattern occurs in Africa varying from 27% in Uganda to 75% in Nigeria. Nearly all toxoplasmosis illnesses in Africa are caused by reactivation of latent infection in HIV related immunosuppression. Cerebral toxoplasmosis or toxoplasma encephalitis (TE) is the main form of the disease resulting from reactivation. The frequency of cerebral toxoplasmosis in HIV varies within Africa, depending on the local pattern of latent infection. In one major HIV autopsy study in West Africa, evidence of cerebral toxoplasmosis was present in15% and was considered the main cause of death in 10%. Toxoplasmosis is considered to be the most common cause of a focal brain lesion in AIDS patients in most parts of Africa.
Clinical presentation
Clinically patients present sub acutely over days or more commonly 1 or 2 weeks with headache and fever often in combination with focal neurological signs. Focal neurological signs occur in around three quarters of patients and include hemiparesis, cranial nerve palsies, ataxia, confusion, altered consciousness and seizures. The pattern of neurologic signs will depend on the site of the focal lesion within the brain and its duration. There may be associated toxoplasma chorioretinitis in 5-10% of affected patients. Cerebral toxoplasmosis occurs mainly in patients with a CD4 count of <100 cells/mm3 and is frequently the first presenting complaint of HIV infection.
Diagnosis
Laboratory investigations are of limited value in the diagnosis. A positive serological screening test for toxoplasmosis usually indicates previous exposure rather than active disease. However a negative result does not exclude the disease. The CSF is also non diagnostic showing predominantly lymphocytes with an elevated protein and a modest decrease in glucose. CT scan of the head with contrast is very helpful for the diagnosis. In cerebral toxoplasmosis, it shows a single or more commonly multiple ring-enhancing lesions with surrounding oedema situated usually in the basal ganglia or/and at the junction of the grey white matter in the cortex (Fig 7.3).
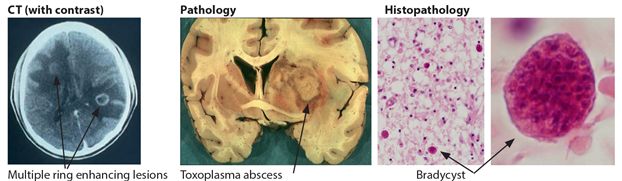
Figure 7.3 Brain in toxoplasmosis
Differential diagnosis
The differential diagnosis of cerebral toxoplasmosis includes other causes of focal neurological disorders in HIV disease, including tuberculoma, primary CNS lymphoma, and rarely progressive multifocal leucoencephalopathy (PML). Other HIV related infections including cryptococcal and TB meningitis may also need to be considered. The clinical presentation of tuberculoma can be very similar to that of cerebral toxoplasmosis, although the clinical course in tuberculoma is usually slower and there may be evidence of concomitant TB elsewhere e.g. chest X-ray. CT features of tuberculoma in adults are those of a single or multiple ring enhancing lesions, with irregular walls of varying thickness and surrounding oedema situated mainly in the cortex. Primary CNS lymphoma is relatively uncommon in HIV in Africa occurring in <1% of HIV patients. The CT in lymphoma may be similar to toxoplasmosis and shows ring enhancing lesions in the deep white matter near the corpus callosum or subependymal areas. In the absence of a CT scan empirical treatment for toxoplasmosis should be started in all HIV patients presenting with focal neurological signs and CD4 count <200 cells/mm³.
Key points
- toxoplasmosis is the most common cause of focal brain lesion in AIDS
- occurs mostly in patients with CD4<100 mm³
- clinical features include headache, fever & focal neurological signs over days or weeks
- diagnosis supported by ring enhancing lesions on CT brain scan
- differential diagnosis includes tuberculoma & lymphoma
Treatment
Treatment is based on drugs that interfere with the ability of T. gondii to synthesise folate and replicate. High dose trimethoprim/sulphamethoxazole (TMP-SMX) is the recommended first line treatment for cerebral toxoplasmosis in Africa (Table 7.3). The dose is one TMP-SMX tablet (80/400 mg) for each 8 kg body weight, taken in two or three divided doses per day. For the average adult this is usually 4 tablets, (1920 mg) twice daily for four weeks, followed by two tablets twice daily for another 8 weeks. Long term secondary prophylaxis with two tablets daily is indicated if the CD4 count is <200/cm3 and should be considered for all patients with CD4 counts < 350 cells/cm3. A clinical response to treatment is usually seen within 3-4 days of starting treatment and definite improvement after 2-3 weeks in over 80% of patients. If there is inadequate or no response to treatment, then other diagnoses other than toxoplasmosis should be considered including tuberculoma. Relapses should be retreated with full course (TMP-SMX). Patients who are intolerant of TMP-SMX because of drug rash or neutropenia can be treated with pyrimethamine 50-75 mg daily in combination with clindamycin 600 mg 8 hourly or azithromycin 1 gram daily. These are continued for a total of 6 weeks. Limiting side effects include drug rashes and bone marrow depression. Folinic acid 15 mg daily should be prescribed with pyrimethamine.
Table 7.3 Drug treatment for toxoplasmosis
| Drug | Dose/route | Duration | Side effects |
| trimethoprim/sulphamethoxazole (TMP-SMX) treatment phase maintenance phase chemoprophylaxis phase | 4 tab (1920 mg) /po/ twice daily 2 tab/po/twice daily 2 tab/po/ daily | 4 weeks 8 weeks until CD4 >350/cm3 | rash, neutropenia |
Prognosis
The mortality in Africa in treated cases of cerebral toxoplasmosis is 10-15% mainly because of late disease and co-morbidity in HIV disease. Relapse rates are considered to be in the order of 20%, occurring up to 6 months after successful treatment. Antiretroviral therapy (ART) should be started within two weeks of starting treatment for suspected or proven toxoplasmosis.
Key points
- treatment is with high dose TMP-SMX for 4/52 & maintenance dose for 8/52
- long term chemoprophylaxis should continue until CD4 is >350 cm3
- ART should start within two weeks of starting treatment
- case fatality rate in treated cases is 10-15%
HUMAN AFRICAN TRYPANOSOMIASIS (HAT)
This is a protozoan parasitic infection caused by parasites of the Trypanosoma brucei, which are transmitted to humans by the bite of the tsetse fly (Glossina spp) (Fig 7.4). Human African trypanosomiasis (HAT), better known as sleeping sickness, is restricted to the distribution of tsetse flies in a vast area throughout sub-Saharan Africa (Fig 7.4). It stretches from 14° north to 20° south latitude, putting a total of over 60 million people in 36 countries at risk. HAT occurs in both epidemic and endemic forms. There are two main types of HAT with differences in epidemiology, biology and clinical features: Trypanosoma brucei rhodesiense (T.b.r.) affects eastern and parts of southern Africa with savannah bush with game animals and cattle as its main reservoir. It accounts for fewer than 5% of reported cases and causes an acute illness. Trypanosoma brucei gambiense (T.b.g.) affects Central and West African river and water hole areas with humans as its main reservoir; it accounts for over 95% of all cases and causes a chronic illness. In Uganda both types of HAT occur, indicating local geographical overlap between Tbr and Tbg infections. The people most at risk live in rural areas and depend on agriculture, fishing, animal husbandry and hunting in African rivers and waterhole areas. HAT has re-emerged during the last decades as a threat to public health in those affected areas. Precise details of disease prevalence are difficult to ascertain. It is estimated that around 300,000 people in Africa are infected with most of those occurring in Southern Sudan and the Democratic Republic of Congo (DRC). Of those approximately 40,000 cases are reported annually to WHO.
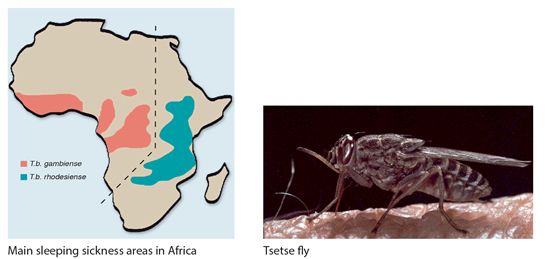
Figure 7.4 Map of trypanosomiasis distribution in Africa
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








