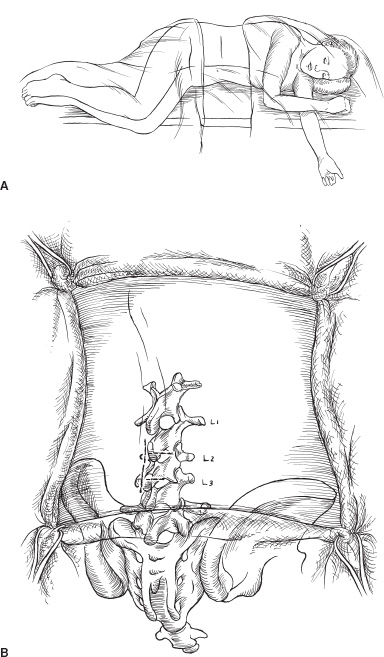
In the past decade, pump insertion for intrathecal infusion of medication has become a relatively common pediatric neurosurgical operation. Pumps are similar to hydrocephalus shunts: Both are implanted devices with the potential to improve quality of life substantially, but both also become infected and malfunction and have catheter problems. As with shunts, meticulous attention to detail during pump insertion will reduce the frequency of complications. Although some pumps are inserted into pediatric patients to treat severe pain, the vast majority are inserted to deliver intrathecal baclofen (ITB) to treat spasticity or dystonia. Briefly, the general indications for ITB in treating spasticity include improving function, facilitating care, and retarding or preventing the development of contractures. Although ITB often improves such abilities as communication and upper extremity function, it is not possible to predict with certainty the ways in which function will improve after spasticity is reduced. Most children or young adults in whom improved function is a goal have spastic quadriparesis, which causes stiff leg movements, limits upper-extremity range of motion, and increases the effort required to use either arms or legs. Patients in whom pumps are inserted to facilitate care are usually seriously retarded and require almost total care. Their spasticity interferes with leg abduction needed for perineal cleaning, knee extension needed to put on pants, and ankle dorsiflexion needed to put on ankle–foot orthoses or shoes. Pumps also are occasionally inserted to prevent or retard contractures, usually in children 4 to 10 years of age whose spasticity is already causing contractures, even if decreased spasticity is unlikely to improve function or facilitate care. Pumps are considered for patients with moderate or severe spasticity, with at least an average muscle tone of 3 on the Ashworth scale. Many younger children will have been treated with oral medications and intramuscular botulinum toxin before pumps are recommended. A trial of oral medications is not a requirement before a pump is considered, however. Some patients have such severe spasticity when first seen, and are alert and cognitively functional, that the likelihood of them obtaining good relief from oral medications is minimal. Most patients with dystonia are given oral medications before proceeding to a pump; but, as with spasticity, those with severe dystonia are unlikely to respond well to oral medications, and a trial of ITB may be the appropriate first treatment. Pumps are rarely inserted without first confirming that the patient responds to ITB. Nowadays, screening trials for spasticity usually are done with a single dose of ITB. Children usually are not sedated for the lumbar puncture because many sedatives reduce their spasticity for several hours and confound interpretation of the test dose. If sedation is needed, intravenous propofol is ideal, providing excellent sedation for less than 1 hour. We apply EMLA cream to the skin of the lumbar region one hour before the lumbar puncture. When the lumbar puncture is done, it is important to measure the opening pressure. Many children with spasticity and cerebral palsy were born prematurely and sustained intraventricular hemorrhages that left them with ventriculomegaly, but they were not shunted. If the opening pressure is 21 cm or greater, I obtain a head scan to evaluate the hydrocephalus. If their pressure is not measured and is higher than normal, their likelihood of developing a cerebrospinal fluid (CSF) leak along the intrathecal catheter is increased. We often continue oral antispasticity medications during the screening trial because they are relatively ineffective. The standard test dose of baclofen is 50 μg; 80 to 90% of patients will respond to it. A clinically significant response is considered to be a 1-point decrease in the average muscle tone of the lower extremities. Tone is graded in the adductors, quadriceps, hamstrings, and plantarflexors before the injection and 2, 4, 6, and 8 hours afterward. Tone is maximally reduced 4 hours after injection. The test dose typically abolishes spasticity in the legs, so it is difficult to stand or walk until the effects begin to diminish. It is not possible to predict during the screening trial what function would be like during continuous infusion. Side effects of headache and vomiting are common during the screening and are related more to the lumbar puncture than to the baclofen. Patients who respond positively to the test dose can be implanted with a pump the following day or soon thereafter. If the response to the 50-μg dose is insignificant, doses of 75 or 100 μg are given. More than 95% of persons with cerebral spasticity will respond to some dose of baclofen. Screening dystonia patients with baclofen differs considerably from screening those with spasticity. If patients have mixed spasticity and dystonia, we try a bolus dose first, but most patients with severe generalized dystonia do not respond to a bolus dose, although they often respond to continuous infusions. We therefore test them with an intrathecal catheter and infuse baclofen with an external microinfusion pump. The catheter is inserted in the operating room under sterile conditions. I first insert a 20-gauge spinal needle in the midline at L2-3 into the epidural space and then insert the 14 to 15 gauge Tuohy needle approximately 5 mm lateral to the midline, insert it into the thecal sac, and advance an intrathecal catheter through it to C5-7, confirming the position fluoroscopically. The guidewire in the catheter is withdrawn, and CSF flow is confirmed. The Tuohy needle is withdrawn, and fibrin glue is injected through the spinal needle into the epidural space to reduce the risk of a CSF leak along the catheter. Then the catheter is tunneled several centimeters laterally, where it exits the skin through a puncture. A pursestring suture is applied at the catheter exit site and a simple stitch at the midline. The catheter is occluded until the patient is on the hospital ward and then is connected to a microinfusion pump. The pump infuses baclofen, initially at a rate of 200 μg per day, and the rate is increased by 50 μg every 8 hours until dystonia in the upper and lower extremities is reduced by 25% or greater on the Barry-Albright dystonia scale, unacceptable side effects such as lethargy occur, or the infusion rate reaches 900 μg per day. Approximately 85% of patients will respond to the infusion, usually at rates of 300 to 600 μg per day. When the infusion trial is completed, if the patient has responded, the catheter exit site is sterilely prepared, the catheter is tied off at the point where it exits the skin, cut off 1 to 2 mm distal to the tie, tucked under the skin, and a stitch is inserted, all so that the catheter may remain in place and be used as the intrathecal catheter when a pump is inserted. If the patient does not respond to the infusion, the catheter is withdrawn and a stitch is inserted. Typically, we send patients home for 2 weeks or longer after the infusion screening trial to ensure that there is no evidence of infection before implanting the pump. Pumps are inserted with the patient under general anesthesia. We use prophylactic antibiotics during the operation and for 48 hours afterward and request that the operative region be washed with an antibacterial soap the night before and morning of operation. Patients usually are placed in the left lateral decubitus position with the right side up; this position is more comfortable for right-handed surgeons and avoids the gastrostomy tubes often present on the left side. Pumps are inserted in the left side if severe scoliosis toward the right narrows the space between the inferior rib margin and the iliac crest. The abdomen, flank, and back are prepared; I prep the skin with Betadine scrub, alcohol, and Duraprep, a mixture of alcohol and iodine that reduces colonization for several hours postoperatively. A transverse incision is made at the abdomen, beginning about 1 cm below the inferior costal margin laterally and extending medially toward the midline for about 3.5 inches (Fig. 25–1). Using the coagulating cautery, subcutaneous tissues are opened down to and through the fascia over the lateral aspect of the rectus muscle, the intervening space, and the external oblique muscle (Fig. 25–2). Fascia is dissected off the muscle cephalad for about 1.5 cm, but mostly inferiorly, for some 8 to 9 cm, so that the pump can be inserted under the fascia without tension. There is no age limitation to pump insertion; the limitation is size: I have inserted a 10-mL Synchromed pump into a 20-pound child, but I insert the 18-mL pump whenever it can be inserted without undue tension. It can be inserted in nearly all children weighing over 40 pounds without difficulty. In patients weighing 30 to 40 pounds, the smaller pump is needed perhaps half the time and in nearly all patients weighing less than 30 pounds. Pumps are secured to the underlying muscle by either eyelets on the pump margins or by Dacron pouches. The eyelets are preferable: they do not tear as the pouches sometimes do, and they do not cause fibrosis, which makes later pump removal difficult. Pumps must be securely sutured to the surrounding tissue. If they are not, they can flip, so that the refill portal is posterior and unaccessible. If a Synchromed pump with a side port is used, I insert the pump so that the side port is caudal; in this way, the catheter attached to the side port is directed superiorly and laterally toward the back (Fig. 25–3).
INDICATIONS
TEST (SCREENING) TRIALS
PUMP IMPLANTATION
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


