Chapter 16 Pupillary and Eyelid Abnormalities
Pupillary Abnormalities
Pupil Anatomy and Neural Control
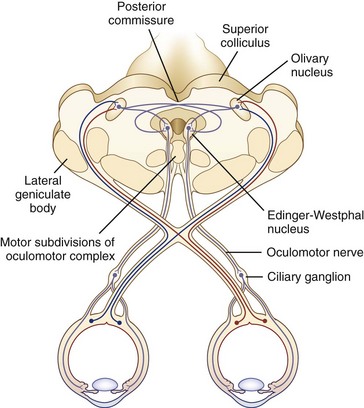
The size of the pupil is determined by the balance of action between two muscles embedded in the iris: the sphincter pupillae, primarily under parasympathetic control, and the dilator pupillae, primarily under sympathetic control. The sphincter is located circumferentially around the pupil and constricts the pupil on exposure to light. The dilator is situated radially and dilates the pupil in darkness. The resting tone of the pupil is primarily determined by the degree of baseline parasympathetic innervation. On exposure to light, the pupil constricts as a result of the pupillary light reflex (Fig. 16.1). The afferent limb of the light reflex originates in the retinal ganglion cells and travels via the optic nerve, chiasm, and optic tract to the dorsal midbrain pretectum just rostral to the superior colliculus, from which neuronal signals are relayed bilaterally to the paired parasympathetic Edinger-Westphal nuclei of the oculomotor nerve (Akert et al., 1980; Nester et al., 2010; Papageorgiou, Wermund, and Wilhelm, 2009). In primate studies, the pretectal olivary nucleus is identified as the primary relay between retinal ganglion cells and the Edinger-Westphal nuclei (see Fig. 16.1) (Kourouyan and Horton, 1997; Pong and Fuchs, 2000; Warwick, 1954). The efferent limb of the light reflex consists of the preganglionic parasympathetic fibers traveling from the Edinger-Westphal nuclei in both oculomotor nerves to the intraorbital ciliary ganglion and the postsynaptic, postganglionic short ciliary nerves carrying the parasympathetic innervation from the ciliary ganglion to the sphincter muscle (see Chapter 70 for a more extensive discussion of oculomotor nucleus and nerve anatomy). The pupillary near reflex consists of pupillary constriction as a response to viewing of a near target. Such miosis is a component of the near triad, along with lens accommodation and ocular convergence. The anatomic substrate of the pupillary near reflex is less well defined than that of the light reflex.
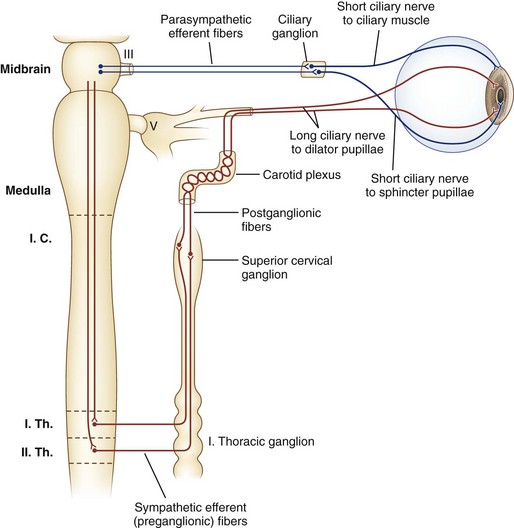
Sympathetic nerves destined for the dilator muscle consist of a chain of three neurons: first-, second-, and third-order neurons (Fig. 16.2). First-order neurons originate in the posterolateral hypothalamus and descend in the dorsolateral brainstem and in the intermediolateral cell column of the spinal cord to the thoracic level (T2). After the first-order neurons synapse in the spinal cord, second-order neurons exit to the paravertebral cervical sympathetic chain via the ventral horns. They ascend near the lung apex and then with the common and internal carotid arteries to reach the superior cervical ganglion in the neck at the angle of the jaw, where they synapse with the third-order neurons. At this point, sudomotor fibers related to facial sweating separate anatomically from those fibers serving pupillary dilation. From the superior cervical ganglion, third-order neurons continue their ascent with the carotid artery through the skull base and into the cavernous sinus, where they temporarily travel with the abducens nerve. They then join branches of the trigeminal nerve, with which they enter the orbital apex, bypass the ciliary ganglion, and reach the dilator muscle via long ciliary nerves (see Fig. 16.2).
Normal Pupil Phenomena
Hippus, or pupillary unrest, is a nonrhythmical, small-amplitude (<1 mm) variation in pupil size that occurs in healthy eyes after light stimulation and is not triggered by accommodation (Hunter et al., 2000; Thompson et al., 1971). After a light stimulus, the pupil constricts, redilates, and then oscillates. The role (if any) of these oscillations in pupillary or visual function is unclear.
With age, pupils become smaller and less reactive to light. Such pupils generally do not require diagnostic evaluation. Although not a normal condition, diabetes similarly affects the pupils sufficiently often as to make small and poorly reactive pupils “normal” in that clinical setting in the absence of any other pathological pupil state. Both parasympathetic and sympathetic pupillary dysfunction are reported in diabetes, and pupillary abnormalities are correlated with a number of other disease processes, including the presence of cardiovascular autonomic dysfunction, peripheral neuropathy, and retinopathy (Bremner and Smith, 2006; Bremner, 2009).
Afferent Pathological Conditions of the Pupils
The relative afferent pupillary defect (RAPD), or Marcus Gunn pupil, is a hallmark of optic nerve disease. It is a manifestation of a relative unilateral disruption of the afferent limb of the pupillary light reflex via the optic nerve and occurs as a result of the consensual and bilateral nature of the light reflex. When a light stimulus is applied to one eye, both pupils constrict as a result of the bilateral connections between pretectal nuclei and the Edinger-Westphal nuclei. When the swinging flashlight test is performed to evaluate for RAPD, the light will be transmitted normally via one optic nerve and to a lesser extent by the diseased optic nerve. This results in the appearance of a brisk bilateral pupillary constriction when the light stimulus is applied to the normal eye and a lesser constriction with initial relative dilation when the light stimulus is transferred to the eye with the optic neuropathy, thus the RAPD. The greater the extent of retinal ganglion cell and optic nerve damage, the larger the relative dilation of the pupil will appear (Lagreze and Kardon, 1998). See Chapter 36 for a more detailed description and a table with step-by-step instructions on how to evaluate for an RAPD, and see Chapter 15 for an extensive discussion of optic nerve disease.
Efferent Pathological Conditions of the Pupils
Clinical Presentation and Examination
The pupil examination (see Chapter 36 for additional discussion) of a patient being evaluated for a pupillary abnormality should begin with observation of the resting positions of the pupils in the ambient room light and of resting eyelid positions. If anisocoria is present, the degree of anisocoria in the light versus in the dark should be evaluated. Pupil evaluation in the dark is done by having the patient look straight ahead in a dark room while the examiner shines just enough light indirectly from below to allow visualization of the pupils. Assessment in the light versus in the dark will help determine which pupil, if either, is the pathological pupil. Anisocoria that is more pronounced in the light suggests that the large pupil is the abnormal pupil, because the small pupil will constrict normally to the light, enhancing the difference in size between the small pupil and the large, nonconstricting pupil. The differential diagnosis for this includes parasympathetic outflow damage (tonic pupil, oculomotor palsy), iris sphincter injury or ischemia, pharmacological pupil dilation, and excessive sympathetic activation. Anisocoria that is more pronounced in the dark suggests that the small pupil is the pathological pupil, because the large pupil will dilate normally in the dark, enhancing the difference in size between the large pupil and the small, nondilating pupil. A caveat to the suggestion that the small pupil is pathological in this situation is that the anisocoria will generally be slightly enhanced in the dark with physiological anisocoria, a normal situation in which neither pupil is pathological (Lam et al., 1987). The differential diagnosis for a pathologically small pupil includes sympathetic inhibition (Horner syndrome), local iris pathology such as inflammation, and parasympathetic stimulation (e.g., pharmacological stimulation).
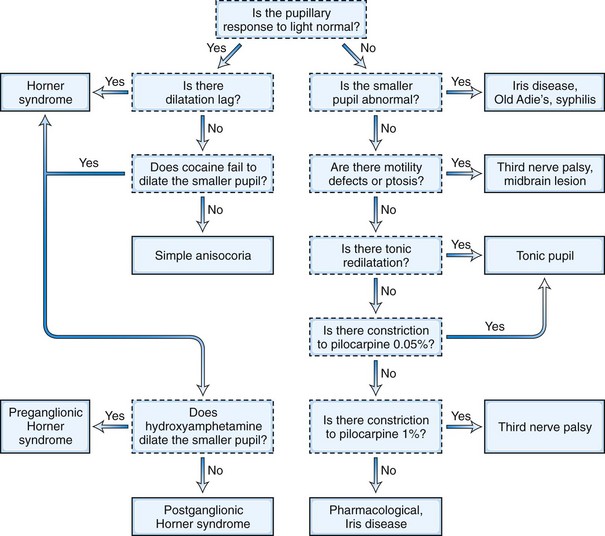
The next step in the examination is evaluation of the direct and consensual pupillary light reflexes, followed by evaluation of the pupillary near response (Kasthurirangan and Glasser, 2006). The near response is best assessed by having the patient hold the thumb several inches in front of the eyes while looking across the room and then to have the patient shift and maintain gaze on the thumb. In certain abnormal conditions, the pupils may have light-near dissociation with poor direct light reflexes but relatively preserved constriction to near stimuli. Once the abnormal pupil is identified, pharmacological testing with a number of topical eye drops is often used for confirmation and assistance in localization (Table 16.1). The general method is application of a drop of the pharmacological agent into each eye, followed by reexamination of the pupils 30 to 45 minutes later. Sensitivity of accurate response detection is increased with before-and-after photographic documentation. Diagnostic use of topical pharmacological agents and additional helpful examination findings for each of the specific disorders are described in detail in the respective sections of this chapter covering the disorders and outlined in a systematic guideline in Fig. 16.3. The presence of a slight degree of ptosis in the eye with the small pupil may indicate sympathetic dysfunction; ptosis in the eye with the larger pupil may indicate oculomotor nerve dysfunction. Careful examination of vision, ocular motility, facial strength and sensation, and ocular fundus should also be performed.
Table 16.1 Diagnostic Pupillary Eyedrop Testing
| Testing | Mechanism of Action | Diagnostic Utility and Expected Response |
|---|---|---|
| ANISOCORIA GREATER IN THE LIGHT (ABNORMAL LARGER PUPIL) | ||
| Dilute pilocarpine (0.0625% or 0.1%) | Parasympathomimetic; direct sphincter stimulation | Tonic pupil will constrict (denervation supersensitivity) |
| Normal pupil and pupil affected by oculomotor palsy or pharmacological blockade will not respond | ||
| Pilocarpine (1%) | Parasympathomimetic; direct sphincter stimulation | Normal pupil and pupil affected by oculomotor palsy will constrict |
| Pupil affected by pharmacological blockade will not respond | ||
| ANISOCORIA GREATER IN THE DARK (ABNORMAL SMALLER PUPIL) | ||
| Cocaine (2% to 10%) | Inhibits norepinephrine reuptake at the sympathetic terminus | Horner pupil will not dilate |
| Normal pupil will dilate | ||
| Hydroxyamphetamine (1%) | Induces third-order sympathetic neuron to release any stored norepinephrine | Preganglionic (first- or second-order neuron) Horner pupil will dilate |
| Postganglionic (third-order neuron) Horner pupil will not dilate | ||
| Apraclonidine (0.5%) | Weak sympathetic agonist | Horner pupil will dilate (denervation supersensitivity) |
| Normal pupil will not change or will constrict slightly |
Anisocoria Greater in the Light
Postganglionic Parasympathetic Dysfunction—Tonic Pupil
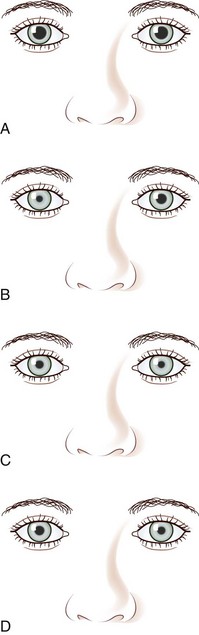
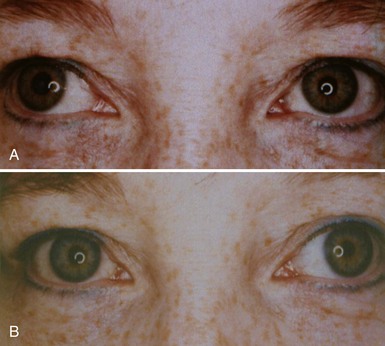
A tonic pupil is large and reacts poorly to light and slowly to near stimuli; after distance refixation, it exhibits a slow, tonic redilation (Fig. 16.4). After removal of the near stimulus, such tonic redilation transiently reverses the anisocoria, making the tonic pupil smaller than the normal pupil, because the normal pupil quickly redilates, and the tonic pupil does not (see Fig. 16.4, D). In the ophthalmology office, evaluation of a tonic pupil at the slit lamp reveals wormlike segmental constriction of some portions of the pupil and absent constriction of other portions. Because of parasympathetic denervation of the iris sphincter muscle, denervation supersensitivity may occur, and instillation of a dilute solution of the cholinergic agent, pilocarpine, may confirm the diagnosis (Fig. 16.5; see also Table 16.1). A 0.1% solution is often suggested, but false-positive results may be more frequent than with a 0.0625% solution (Ashker et al., 2008; Leavitt et al., 2002). Dilute pilocarpine will constrict the tonic pupil, but not a normal pupil or a dilated pupil from another cause such as oculomotor palsy or pharmacological toxicity. Solutions of pilocarpine less than 1% are not commercially available and must be prepared by dilution with preservative-free normal saline solution.
Tonic pupils result from damage to the intraorbital ciliary ganglion or short ciliary nerves from any etiology, including focal infectious (herpes zoster, syphilis) or noninfectious (giant cell arteritis) inflammation, malignant infiltration, paraneoplastic processes, and trauma. Tonic pupils may also be seen as a component of a systemic autonomic neuropathy (see Chapter 77) (Bremner and Smith, 2006; Yamashita et al., 2010), Guillain-Barré syndrome or its Miller Fisher variant, and botulism. Perhaps the most common and most easily recognizable tonic pupil is the benign Holmes-Adie pupil, which often presents with acute painless enlargement of the pupil and may be accompanied by complaints of photophobia and blurred near vision due to involvement of fibers serving the ciliary body for lens accommodation. In 80% of patients, the condition is unilateral. It is most common in healthy young women and is thought to be due to a viral ciliary ganglionitis. Additional examination findings may include decreased deep tendon reflexes and decreased corneal sensation. The affected pupil tends to become smaller over time and may even eventually become smaller than the unaffected pupil. Ross syndrome is the triad of tonic pupils, hyporeflexia, and segmental anhidrosis (Ross, 1958). It is unclear whether this syndrome is a variant of the Holmes-Adie syndrome or a mechanistically distinct disorder, but impaired thermoregulation is the distinguishing element (Nolano et al., 2006; Shin et al., 2000). Harlequin syndrome, failure of facial flushing to thermal or emotional stress, may also occur with tonic pupils and hyporeflexia, although Horner syndrome is more common (Bremner and Smith, 2008).
Preganglionic Parasympathetic Dysfunction—Oculomotor Palsy
Pupillary enlargement from oculomotor palsy may be accompanied by ocular motor deficits due to impairment of the oculomotor-innervated medial, inferior, and superior rectus and inferior oblique muscles, and by ptosis due to involvement of the levator superioris muscle. Isolated pupillary involvement may occur as an early sign of neurovascular compression by a posterior communicating artery aneurysm because the pupillary fibers are located superficially on the superomedial surface of the nerve. It may also be a sign of herniation of the temporal lobe uncus from increased intracranial pressure, known as Hutchinson pupil (Ropper, 1990). A fixed and dilated Hutchinson pupil on initial presentation of head trauma, stroke, or intracranial mass lesion is associated with 75% mortality (Clusmann et al., 2001). Although dilute pilocarpine (0.1%) does not typically result in constriction of the pupil in this situation, it should constrict after administration of 1% pilocarpine (see Table 16.1). This may help in differentiating isolated pupillary enlargement from an oculomotor palsy from pharmacological pupillary enlargement, because the pharmacological pupil should not constrict. See Chapter 70 for a complete discussion of oculomotor nerve anatomy and clinical lesions; Fig. 70.2 shows an example of oculomotor palsy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree