Chapter 123 Retrogasserian Glycerol Rhizolysis in Trigeminal Neuralgia
History
The discovery of the beneficial effects of glycerol in patients with TN was purely accidental. During the course of development of a procedure for producing lesions in the gasserian ganglion in patients with TN in the 1970s, in which the Leksell gamma knife in Stockholm was to be used, x-ray contrast medium (metrizamide) and glycerol were tried as vehicles for a radiopaque metal dust (tantalum powder). The tantalum powder was to be introduced into the retroganglionic cistern as a permanent marker to constitute a visible target for the subsequent stereotactic calculations.1,2 Glycerol was chosen as the vehicle because, being the base for triglyceride formation in the body, it was presumed to be harmless, and its viscosity would ensure that the tantalum suspension was maintained long enough for the powder to be deposited in the trigeminal cistern. In fact, glycerol had been used earlier in the treatment of TN as a vehicle for the highly neurolytic phenol,3 which was used for percutaneous treatment of TN at that time. It was noted that merely injecting the glycerol and tantalum dust mixture in patients abolished paroxysmal pain before the gamma knife procedure was performed. On the basis of these observations, Håkanson developed the technique for treating TN by glycerol injection into the trigeminal cistern. The first series of patients was presented in 1981,4 and the method was then rapidly adopted in many neurosurgical centers.
Over the years, many series of patients treated using Håkanson’s procedure, or some variation of the original method, have been reported. The results from different series have been highly variable. In many centers the outcome has been quite satisfactory,5–9 and glycerol rhizolysis has continued to be the method of choice, particularly for elderly and infirm patients. In other series, the results have been so discouraging (Siegfried, 1985, and Rhoton, 1985, unpublished results, both cited in Sweet10; Price, 1985, unpublished results, cited in Sweet11 and Fujimaki and colleagues12) that some neurosurgeons have entirely abandoned the procedure.
Probable Mechanisms of Action of Glycerol
In the literature we find no satisfactory animal models of trigeminal neuralgia, and it is difficult to obtain relevant histologic data from patients. However, trigeminal neuralgia presents with such idiosyncratic signs and symptoms, and responds to so distinctive a set of therapeutic modalities, that scientific deduction can be used to generate likely hypotheses. The “ignition hypothesis” of trigeminal neuralgia13–15 is based on advances in the understanding of abnormal electrical activation of injured sensory neurons,16 supported by histopathologic examinations of biopsy specimens from patients with trigeminal neuralgia who are undergoing microvascular decompression (MVD) of the trigeminal root in the posterior fossa.13 According to this hypothesis, trigeminal neuralgia results from specific abnormal activation of trigeminal afferent neurons in the trigeminal root or ganglion. Injury renders both axons and axotomized somata hyperexcitable. The hyperexcitable afferents, in turn, induce pain paroxysms as a result of synchronized after-discharge activity. The ignition hypothesis accounts for the major positive and negative signs and symptoms of trigeminal neuralgia, for its pathogenesis, and for the efficacy of treatment modalities (for discussion, see Devor et al. 14 and Rappaport and Devor15).
Morphologic Effects of Glycerol
Glycerol is a trivalent alcohol normally present in human tissue, where it forms the skeleton of the triglycerides, among other functions.17,18 Glycerol readily penetrates cell membranes and seems to possess distinct cryoprotective properties beneficial to cells. Its toxicity is low, and comparatively high doses must be injected systemically or intrathecally to induce toxic effects.19,20 Glycerol’s neurolytic action is thought to be due to its hypertonicity, a condition known to injure nerve fibers, especially thin, unmyelinated, and myelinated fibers.21 Although the myelin sheath of the coarse fibers gives some transitory protection from this effect, length of exposure, neuron type, and the presence of previous demyelination may be important determinants of the vulnerability of individual fibers. For example, with longer exposures, Robertson22 and Pal and colleagues23 observed that myelinated fibers were particularly vulnerable, and the degree of damage positively correlated with fiber diameter.
Studies on isolated animal nerve fibers show morphologic changes after exposure to glycerol. These consist of disruption of the tight junction between the Schwann cells and the axolemma, without damage to the axon proper.24 Bathing the fibers in glycerol initially causes the axons to shrink, with a return to basal volume after equilibration of the substance over the cell membranes. With transfer to iso-osmotic conditions, the fibers swell markedly before returning to their normal volume. Thus, marked structural changes are observed with glycerol administration, but the conduction properties of the treated nerve axons remain intact.24
After intraneural and perineural injection of glycerol, Håkanson25 and Rengachary and associates26 observed axolysis with marked myelin sheath swelling. The coarse myelinated fibers sustained the most severe damage, whereas the small-diameter myelinated and unmyelinated fibers are relatively well preserved.23 In contrast, Bremerich and Reisert27 found only slight histomorphologic changes after glycerol injection in the region of the foramen ovale in the rat in their long-term (180 days) comparative study of axonal damage after injection of glycerol, phenol-glycerol, and saline. A more recent study in dogs submitted to glycerol injection in a single trigeminal ganglion28 demonstrated axonolysis both in myelinated as well as in nonmyelinated fibers.
The damage following glycerol injection into a cavity with isotonic body fluid is probably considerably less severe than perineural deposition. However, Lunsford and associates9 observed extensive areas of myelin degradation and axonal swelling in cats subjected to retrogasserian glycerol injections 4 to 6 weeks earlier.
The site of glycerol effects has been specifically studied by Stajcic,29 who injected 3H-labeled glycerol into peripheral branches of the maxillary nerve and in the infraorbital canal of rats. The amount of radioactivity detected in the nerve distal to the foramen rotundum, as well as in the ipsilateral and contralateral gasserian ganglion, was less than 0.1% in all specimens. The author concluded that a retrograde transport mechanism behind the effect is improbable and that the beneficial effect of glycerol occurs at the site of injection.
There is as yet no publication of an autopsy series of patients with TN treated by retrogasserian glycerol rhizolysis. Sweet11 provides an anecdotal description of a patient undergoing a retrogasserian glycerol injection of the extreme volume of 1.5 ml, with subsequent development of anesthesia dolorosa. At a posterior fossa craniotomy “many months” later, the trigeminal rootlets were found to be markedly atrophic.
Neurophysiologic Changes after Glycerol Application
Burchiel and Russel30 studied the effect of glycerol on normal and damaged nerves in a rat neuroma model. The neuromas, produced by sectioning of the saphenous nerve, were mechanosensitive and discharged both spontaneously and in response to light manipulation. These researchers found evidence supporting the view that glycerol exerts its major action on the large-diameter fibers. Exposure of the injured nerve to glycerol induced a short episode of increased spontaneous firing in the nerve, a response shown to originate from the myelinated fibers.
The observation by Rappaport and associates31 that glycerol injected into neuromas was more effective than alcohol in decreasing autotomy in rats suggests that autotomy may be related to unpleasant “tic-like” paresthesias. The therapeutic mechanism, according to these investigators, could be suppression of ectopic impulse barrage from the neuroma.
Sweet and co-workers32 found that glycerol injected into the trigeminal cistern of patients abolished the late components (corresponding to A-delta and C fibers) of trigeminal root potentials recorded with electric stimulation of the surface of the cheek. These recordings were made only minutes after the injection, and therefore do not permit conclusions concerning long-term effects.
Hellstrand and colleagues (unpublished data; see Håkanson25) studied the effects of glycerol both on isolated frog nerve and on trigeminal root fibers after cisternal injection in the cat. They observed a severe reduction of the evoked potentials with glycerol but a nearly total restoration after rinsing the compartment with saline. This recoverability probably has a bearing on clinical effects and must be taken into account when interpreting the short-term observations of Sweet and associates32 referred to previously. Based on knowledge that glycerol requires at least 30 minutes to equilibrate across a membrane of a living cell and according to the aforementioned experimental observations, evacuation of the glycerol from the cistern after a short time (e.g., 5 to 20 minutes10,33,34) might induce more severe damage, especially to fine fiber systems, than a slow unloading by diffusion into the subarachnoid space.
Longer-term observations of trigeminal evoked potentials have been reported by Bennett and Lunsford,35 who investigated patients before and 6 weeks after trigeminal glycerol rhizolysis. They confirmed the earlier findings of Bennett and Jannetta36 that thresholds were elevated and evoked potentials had a markedly increased latency on the affected side compared with the healthy one. An additional, unexpected finding was that these aberrations were “normalized” after glycerol rhizolysis. Because partially demyelinated fibers are known to conduct with a slower velocity and at a lower rate,37,38 they interpreted this finding to indicate that glycerol selectively attacked partially damaged trigeminal axons and, after their elimination, the evoked trigeminal potentials appeared “normalized.”
Further long-term observations were supplied by Lunsford and colleagues,9 who noted the most marked changes in trigeminal evoked potentials in cats in the large-diameter myelinated fibers, with additional changes noted as late as 6 weeks after the injection.
Quantitative sensory testing using von Frey hairs, mechanical pulses, and the Marstock technique39 also corroborates the notion that glycerol acts mainly on the large myelinated fiber spectrum.40 Eide and Stubhaug41 examined thresholds for tactile and temperature stimuli in patients with TN before and after glycerol rhizolysis. They found evidence that pain relief after glycerol treatment involved normalization of previously abnormal temporal summation phenomena with little accompanying sensory loss. Kumar and associates42 found postinjection quantitative abnormalities of the blink reflex that correlated with sensory impairment.
Thus experimental and clinical observations indicate that the effects of glycerol may be due to its hyperosmolarity and that the rate of alteration of osmolarity is critical for the effect. Furthermore, there are indications that the major part of the effect is exerted through actions on large myelinated fibers, notably those with previous damage to the myelin sheath, thereby possibly affecting the “trigger mechanism” for pain paroxysm. Glycerol has also been reported to downregulate central neuronal hyperexcitability, often without signs of significant additional nerve damage.41
Technique
The original technique of Håkanson has been subject to many modifications by various neurosurgeons. These variations encompass the type of anesthesia selected, general or local; patient position and fluoroscopic projection; whether cisternography is performed; other modes of localization of the needle tip (electric stimulation; reactions to drop-by-drop injection of glycerol, local anesthetic injection43); the dose of glycerol used; instillation of glycerol in one step or as minute volumes in an incremental fashion, with intermittent sensory testing; trials to empty the cistern after attaining a satisfactory effect according to perioperative testing; and how long the patient is kept sitting with the head flexed after completion of the procedure.
Some of these modifications have resulted in less satisfactory results.10,12,33,44 We consider retrogasserian glycerol rhizolysis to be an anatomically based method aimed at graded lesioning of fibers in a certain locus. Thus the localization procedure should also be anatomic and the treatment should be meticulously performed, using the smallest possible volume of pure, sterile glycerol considered to be effective in each case.
Anatomic Landmarks and Important Structures
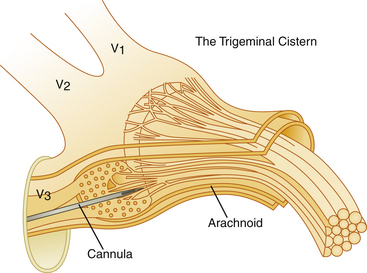
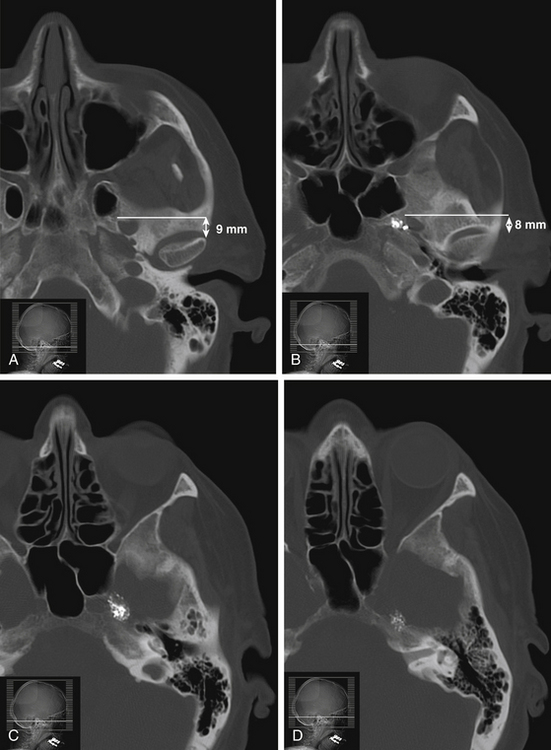
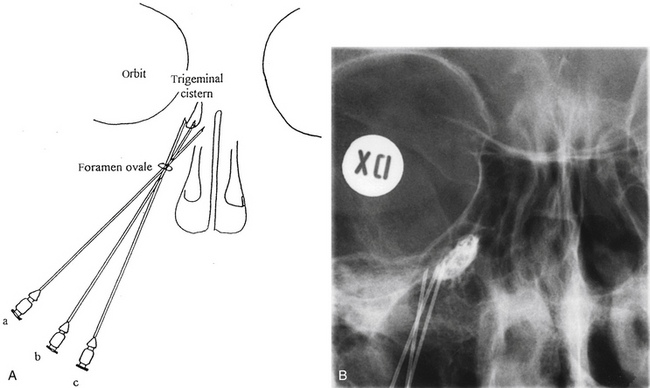
The trigeminal cistern is punctured by the anterior percutaneous route through the foramen ovale, as described by Härtel45 (Fig. 123-1). After local anesthesia, a 22-gauge lumbar cannula (outer diameter 0.7 mm; length 90 mm) is inserted from a point approximately 3 to 4 cm lateral to the corner of the mouth. The trajectory is aimed at a point that lies, in the lateral view, approximately 0.5 cm anterior to the anterior margin of the mandibular joint, and in the anteroposterior view, toward the medial margin of the pupil with the eyeball in the neutral position. There are several landmarks that may be used for reaching the foramen ovale,46,47 but in most cases these two coordinates are sufficient. In Fig. 123-2, CT scans from a patient with tantalum dust in the trigeminal cistern are provided to show the relationship of the oval foramen and the trigeminal cistern to these structures (Fig. 123-2 A through D).
When the tip of the cannula is located inside the arachnoid of the trigeminal cistern, there should be a spontaneous exit of cerebrospinal fluid (CSF), especially at the first treatment. Because the location of the trigeminal ganglion and cistern can vary in relation to the landmarks of the skull base, a contrast injection must be performed to ascertain the correct site for glycerol injection. However, spontaneous CSF drainage is not sufficient for accepting the location as intracisternal, as CSF may originate from other locations; in fact a brisk flow of CSF often indicates a subtemporal tip location.
Trigeminal Cisternography
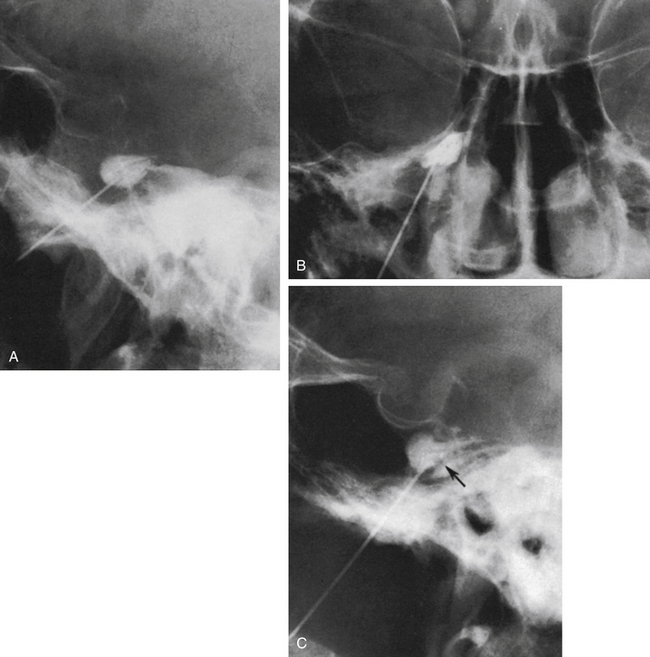
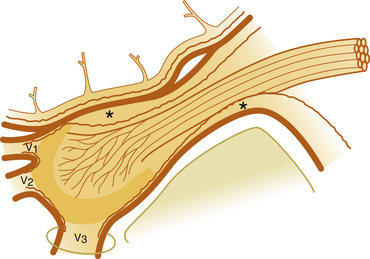
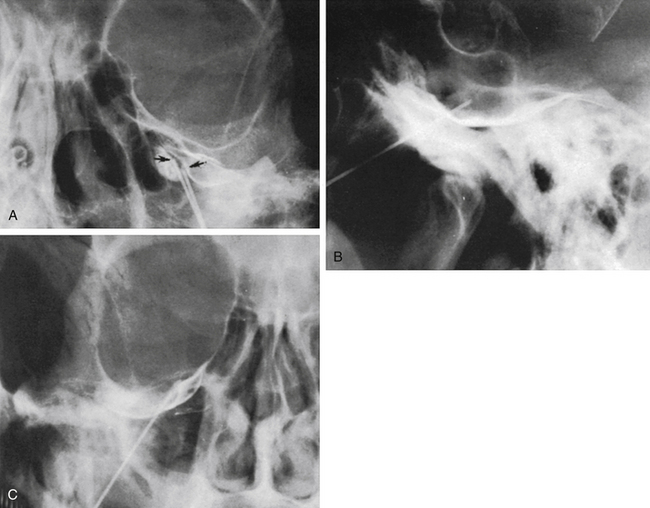
The technique we use is essentially the same as that described by Håkanson,48 although estimation of the cisternal volume is of less importance for deciding what volume of glycerol to inject. The contrast medium must be water soluble, with high radiographic attenuation and low toxicity, and must have a higher specific gravity than CSF. The contrast medium used since 1986 is iohexol (300 mg iodine/ml).49 Approximately 0.3 to 0.6 ml is injected with the patient sitting with his or her head slightly flexed to retain as much of the medium in the cistern as possible. If intermittent fluoroscopy is used during injection, the position of the needle tip may be estimated immediately, but it should always be confirmed by radiography in both the lateral and anteroposterior projections. The typical appearance of the trigeminal cistern is illustrated in Fig. 123-3. Ideally, the sensory root filaments (and sometimes the motor portion) should be visualized by lateral cisternography, leaving no doubt about the intracisternal position of the tip. The typical 45-degree medial tilt of the cistern should be seen from the anteroposterior view (Fig. 123-3B). The appearance of the cistern may vary considerably between patients, and it is essential that the surgeon be familiar with this anatomy. Furthermore, there is a subdural-extracisternal compartment in Meckel’s cave that may be injected with contrast medium (Fig. 123-4). This usually happens when contrast medium is injected without prior spontaneous CSF drainage, or if the needle is dislodged from the intracisternal position during injection.
Specific Difficulties
Spontaneous CSF drainage from the needle does not guarantee an intracisternal tip location. In fact, if the cannula is placed a few millimeters too lateral, the tip may be located in the subtemporal subarachnoid space, and a flow of CSF will still be produced. Subsequent cisternography solves this problem. Figure 123-5A shows the proximity of the cisternal and subtemporal subarachnoid compartments in the anteroposterior projection. Figure 123-5B and C illustrate a pure subtemporal contrast injection. Our strategy in this case is to leave the first needle in place and to introduce a second needle using the first one for guidance (Fig. 123-6A and B). It cannot be overemphasized that spontaneous CSF drainage is required before contrast injection. If, in such a case, the contrast medium is injected without fulfilling this requirement, the glycerol may be deposited in the extra-arachnoid, subdural compartment as shown above (see Fig. 123-4).
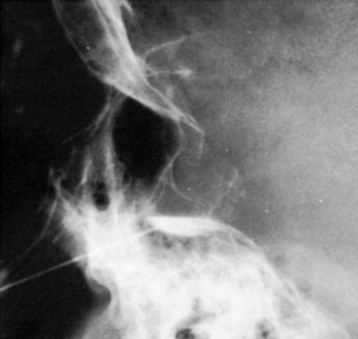
Another problem may arise if the patient’s head is not adequately tilted forward during the injection, because the contrast medium then flows out the porus trigemini and escapes to the posterior fossa, with insufficient cisternal filling to confirm the correct position of the needle tip (Fig. 123-7). This situation may also result in an underestimation of the cisternal volume.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree