Fig. 35.1
A 17-year-old female with painful “adding on” below prior posterior selective thoracic spinal fusion for adolescent idiopathic scoliosis. Following the index surgery, she experienced progressive spinal deformity and pain symptoms throughout the spine and into the lower extremities that interfered with her ability to sit, stand, or walk for more than a few minutes. She was neurologically intact and otherwise very healthy. (a) A anteroposterior radiograph taken prior to the revision deformity surgery demonstrating a right thoracic curve of 60°, a left lumbar curve of 50°, and instrumentation T4–T11. Diagnosis of “adding on” below the prior fusion was made, and the patient was indicated for revision surgery for removal of instrumentation, posterior osteotomies (Smith-Petersen distally) to allow correction, and distal extension of posterior instrumented fusion to L4. The patient underwent the planned procedure with use of an ISOLA hybrid construct and distal pedicle screws, along with right-sided thoracoplasties and iliac crest bone graft harvest. By two months after the revision surgery, she required no pain medicine, and preoperative symptoms had resolved. (b) A 6-month postoperative anteroposterior radiograph showing revision instrumentation and deformity correction to 25° in the thoracic and 20° in the lumbar spines. She remains symptom-free in long-term follow-up and is managed by a physician located closer to her home
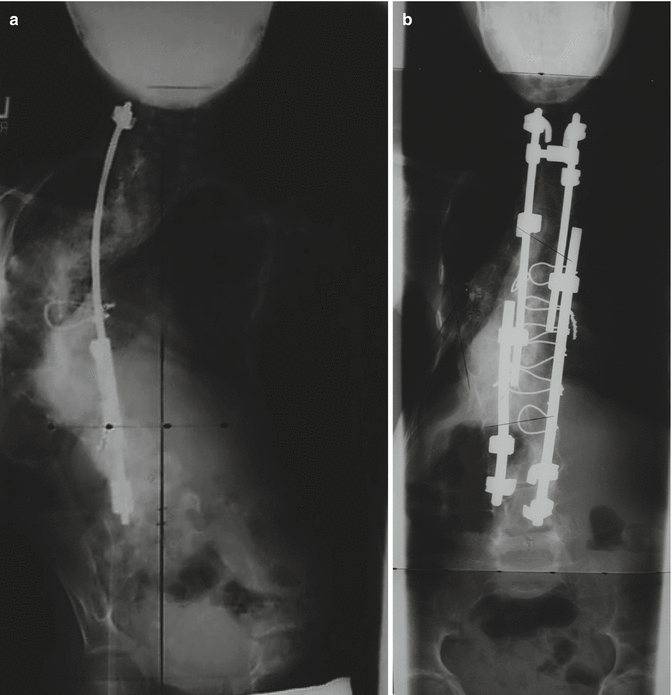
Fig. 35.2
A 7-year-old female with Marfan’s syndrome diagnosed with scoliosis at 3 years old, who had undergone bracing and growing rods and then had loss of proximal implant fixation and crankshaft phenomenon after definitive fusion. Revision operative management was slightly delayed due to her emaciated state and need for nutritional optimization by gastroenterology consultation, along with placement of a gastrostomy tube and enteral supplementation. (a) An anteroposterior radiograph taken prior to the revision surgery showing loss of proximal fixation, main thoracic apex left curve from T5 to T12 measuring 128° and a 35° curve from T12 to L5. After application of preoperative traction, the T5–T12 curve was reduced to 108°. She had global kyphosis of 60°, was Risser 0, and had 3-dimensional imaging demonstrative of dural ectasia. She was indicated for revision anteroposterior fusion with posterior instrumentation and underwent anterior osteotomies from T5 to T12 and a staged posterior procedure. During the second-stage posterior procedure, multiple Smith-Petersen osteotomies were performed, and an ISOLA four-rod construct was used to instrument from T2 to L4 with claw and wire fixation. (b) A postoperative anteroposterior radiograph showing the revision instrumentation and correction of deformity with residual thoracic scoliosis of 40°. At most recent follow-up seven years after the revision surgery, she had gained 8 in. in height and 24 lb in weight and was pain-free and neurologically intact
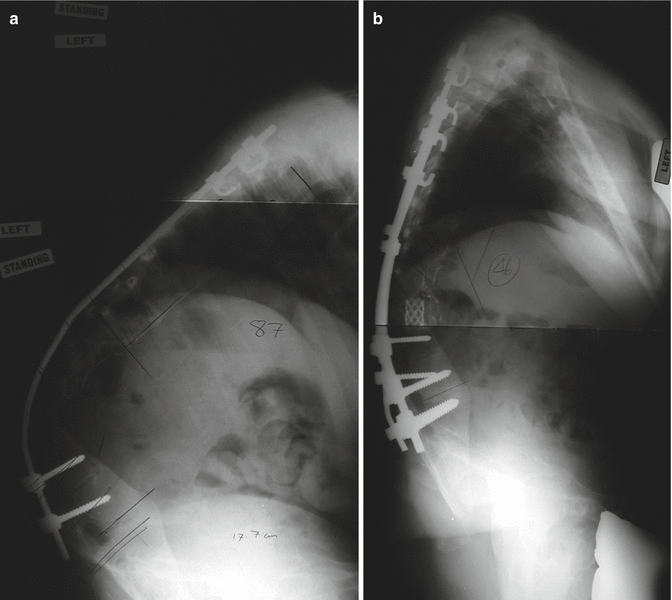
Fig. 35.3
A 13-year-old female with progressive kyphosis after “growing rods.” She was diagnosed with neurofibromatosis type 1 at age 2 years and began growing rod management at age 3.5 years. She had 11 surgeries prior to presenting to our institution, the last of which involving infected implants, requiring implant removal and deformity management with a TLSO appliance. (a) A standing lateral radiograph taken prior to the placement of halo traction showing T3–L1 kyphosis of 125° with apex at T8/T9. She underwent a staged procedure where a halo fixator was applied; she had 3 months of axial traction (50 % of body weight), where the T3–L1 kyphosis decreased to 100°; and she underwent T2–L3 posterior spine fusion with instrumentation, including a T8–T10 posterior vertebral column resection. (b) A standing lateral radiograph taken at 1 year postoperatively following PSFI with PVCR demonstrating revision posterior instrumentation, anterior titanium mesh cage, posterior titanium mesh connected to the rods preventing soft tissue encroachment on the neuroelements, and overall kyphosis measurement of 55°. She had physiological coronal and sagittal balance, was neurologically intact, had no pain symptoms, and had returned to participation in her school play
The type of revision surgical intervention was determined on a case by case basis, with 25 patients receiving anteroposterior fusions and three being treated with posterior spinal fusions. Instrumentation was used in all cases. Surgical revision for the eleven crankshaft phenomenon patients was anteroposterior fusion, as dictated by their disease. The pseudarthrosis patients were also managed with anterior spinal fusion to best guarantee solid arthrodesis across the segment and posterior instrumented fusion for correction and compression across the segment. Additional anterior procedures were indicated for osteotomy of unacceptable residual deformity and to mobilize the spine for improved correction. Stand-alone posterior spinal fusions were performed as a simple extension in one patient and were combined with posterior osteotomies in two patients. Postoperative correction in the coronal plane was from 67° (range, 32–115°) preoperatively to 36° (range, 20–60°) postoperatively, for a mean correction of 43 % (range, 17–66 %). Sagittal measurements in the thoracic spine had a mean of 57° (range, 20–107°) of kyphosis preoperatively and 51° (range, 20–95°) postoperatively and in the lumbar spine were 66° (range, 40–106°) of lordosis preoperatively and 60° (range, 40–88°) postoperatively.
Major complications occurred in three patients and included one superficial wound dehiscence, one pleural effusion/pneumothorax, one junctional kyphosis, and one proximal hook implant dislodgement. Each of these required invasive intervention, and each patient eventually fully recovered. No events of neurological deficit, deep infection, or death occurred. No patients required placement of tracheostomy, despite nine patients having preoperative forced vital capacity averaging 30 % of predicted (range, 20–40 %). None of the 28 patients have required further spine surgery.
35.5 Conclusions
Revision spine surgery in the growing child is technically challenging and not encountered frequently except in growing rod patients. In approaching this complex problem, the surgeon must have a complete understanding of the primary deformity and reason for index surgery failure, comprehend the residual growth potential of the spine and how this affects the revision surgery plan, and have a realistic appreciation of technical ability required to perform the procedures safely. Remember: first do no harm, and if in doubt, do not hesitate to refer the patient to a more experienced surgeon or to ask for help. Indications and goals for revision surgery need to be agreed upon by the surgeon and the family of the child to assure that postoperative satisfaction is maximal, and preoperative planning must include a multidisciplinary evaluation to optimize both care delivered and surgical outcome.
References
1.
Auh JS, Binns HJ, Katz BZ (2004) Retrospective assessment of subacute or chronic osteomyelitis in children and young adults. Clin Pediatr (Phila) 43:549–555CrossRef
2.
Berven SH, Deviren V, Smith JA, Hu SH, Bradford DS (2003) Management of fixed sagittal plane deformity: outcome of combined anterior and posterior surgery. Spine 28:1710–1715; discussion 1716PubMed
3.
4.
5.
6.
Bradford DS, Tribus CB (1994) Current concepts and management of patients with fixed decompensated spinal deformity. Clin Orthop Relat Res 306:64–72PubMed
7.
Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K (2003) Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am 85-A:454–463PubMed
8.
Bridwell KH, Lewis SJ, Rinella A, Lenke LG, Baldus C, Blanke K (2004) Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance surgical technique. J Bone Joint Surg Am 86-A(Suppl 1):44–50PubMed
9.
Bunnell WP (1988) The natural history of idiopathic scoliosis. Clin Orthop Relat Res 229:20–25PubMed
10.
Campos M, Dolan L, Weinstein S (1976) Unanticipated revision surgery in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 37:1048–1053CrossRef
11.
Cho KJ, Bridwell KH, Lenke LG, Berra A, Baldus C (2005) Comparison of Smith-Petersen versus pedicle subtraction osteotomy for the correction of fixed sagittal imbalance. Spine 30:2030–2037; discussion 2038CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








