Chapter 105 Role of Gamma Knife Radiosurgery in the Management of Arteriovenous Malformations
Following Roentgen’s discovery of x-rays, there was early interest in the use of irradiation for arteriovenous malformation (AVM) treatment from 1914 to 1950.1 In spite of occasional successes, until 1970, there was consensus among neurosurgeons that radiation had little practical use in the management of AVMs.2
Things began to change with the development of the concept of stereotactically guided treatment of intracranial lesions using a single high dose of ionizing beams by Lars Leksell.3 He defined the term and initiated investigation, first using an orthovoltage x-ray tube combined with his initial rectilinear stereotactic coordinate frame. In 1951, working with Borje Larsson, a physicist, Leksell subsequently reported the first clinical use of stereotactic guidance linked with a cyclotron-generated proton beam.4
In 1968, continuing their work, Leksell built the first dedicated radiosurgical unit, the gamma knife.4,5 The potential value of irradiation in the treatment of vascular malformations was subsequently reassessed. Encouraging factors were evidence that the vessels were radiosensitive and a long-term angiographic follow-up of a small series of AVMs treated with fractionated irradiation by Johnson in the 1950s confirming that the AVM was obliterated in 45% of cases.6
Stereotactic radiosurgery is the closed skull destruction of an intracranial target using ionizing beams of radiation, directed in a focused manner with the help of an extracranial guiding device.7 The application of stereotactic radiosurgery for cerebral arteriovenous malformations began in earnest in the early 1970s.10
Radiosurgical Treatment of Arteriovenous Malformations
It has been estimated that as many as 500,000 people in the United States and Canada have AVMs.8,9 During the last 40 years, the overall role of stereotactic radiosurgery has been established in the management of selected vascular malformations. Together with advances in microsurgical techniques, postoperative neurointensive care management, and advances in intravascular microcatheter techniques for AVM characterization and embolization, radiosurgery alone or in combination represents a moderately low-risk and effective strategy for properly selected patients.11,12
A cure for every patient treated with radiosurgery has not yet been achieved. The obliteration rates at 3 years (“angiographic cure”) range from 70% to 85%.13–17 Several factors have been associated with the likelihood of obliteration, such as AVM volume, prescription dose, location, angioarchitecture, and patient age and lack of history of embolization. Radioresistance has been reported in some lesions that show no response, despite appropriate doses of radiation.17,19
Small treatment volume, a 23- to 25-Gy prescription dose, and a small number of draining veins18–23 are factors associated with a more favorable prognosis. Conversely, factors such as a large AVM’s volume, site, and the radiation dose to the exposed brain tissue, have been associated with the likelihood of complications, including bleeding during the latency period, cerebral edema, and/or radionecrosis.23–34
Some researchers, including Chang et al.,35 have suggested that other factors in addition to volume should be considered when predicting obliteration results, such as the AVM’s flow pattern and the number of draining veins. Furthermore, some lesions that appear to be similar in vessel architecture, location, and volume, respond differently or unexpectedly to treatment plans based on the dose–volume relationship. Some AVMs are obliterated within 2 years, whereas others do so after 3 or 4 years.
In terms of angiographic features associated with lower likelihood of obliteration, Meder et al.36 reported that radiosurgery is less effective in AVMs with direct intranidal fistulas and single draining vessels and suggest that radiosurgery is more likely in patients with plexiform or compact-type AVMs.
Similarly, in a semiquantitative flow study using phase-contrast two-dimensional (2D) magnetic resonance angiography (MRA), Petereit et al.37 reported a negative effect on the obliteration of AVMs by flow speeds greater than 100 ml/s. We reviewed our own experience by defining a critical zone for treatment, the high flow nidus excluding the perilesional draining veins in AVMs using dynamic analysis of circulation times through the AVMs.
Improved Imaging in Radiosurgery
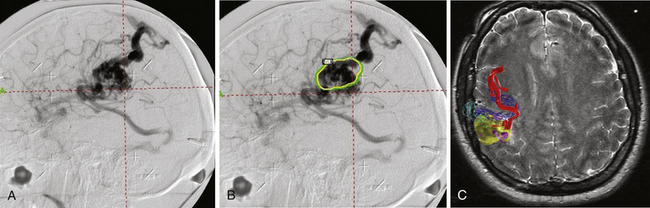
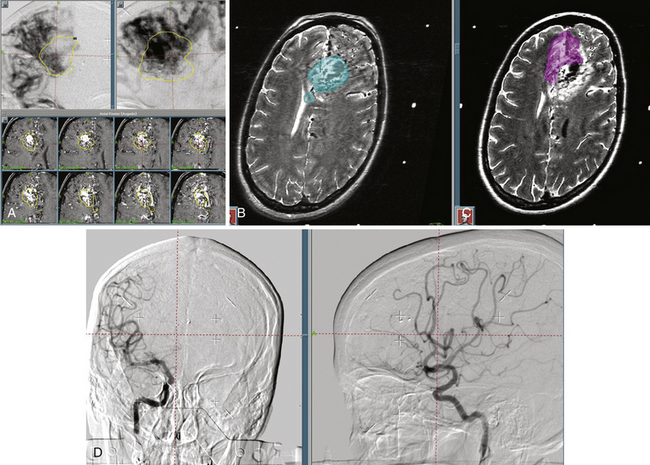
The accuracy of radiosurgical treatment is dependent on the accuracy of target delineation by radiological studies performed with a stereotactic frame in place. In the management of vascular malformations, the primary imaging modality is angiography. Magnetic resonance imaging (MRI) examinations are also recommended for obtaining further information, to fully understand the topographic and tomographic anatomy of the malformation as well as the relationship between the targeted AVM and the non-targeted anatomical structures adjacent to the AVM. The gamma surgery planning program allows target outlines marked on the angiogram to be projected on the MRIs and vice versa (Fig. 105-1). Thus, both modalities can be used in the planning procedure.
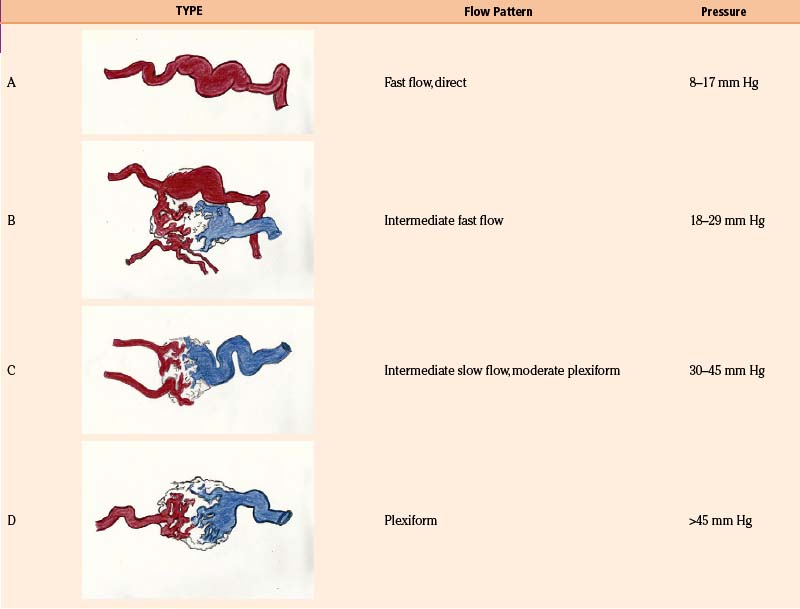
With the goal of improving imaging, and therefore targeting of the AVM, our protocol focuses on the definition of the AVM’s critical zone, which corresponds to the least-resistant portion of the lesion with the predominant flow draining to the main vein. Our experience in measuring the pedicular pressure in feeders, when treating malformations with superselective embolization, was taken into account in a dynamic study of malformations.38 Pedicular pressures lower than 25 mm Hg are observed in direct AV fistulas, whereas plexiform AVM pressures increase gradually to nearly 50 mm Hg (Table 105-1).
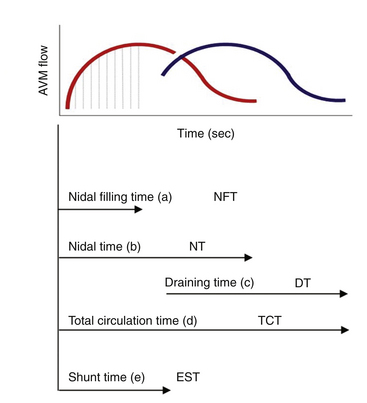
In our protocol, once the Leksell stereotaxic frame (Elekta) is in place on the patient’s head, three-dimensional (3D) volume MRIs are acquired in T1 simple and gadolinium-enhanced sequences, together with axial T2 and coronal sequences for 3D images of the AVM. For digital subtraction angiography (DSA), Axiom Artis equipment is used with software for automated outline correction (Artis&Syngo, Siemens). Currently, images are obtained at 10.5 frames/seconds with a digital contrast injector (Mark V Pro Vis, Medrad), with contrast injected at 7 ml/s in the proximal portion of the carotid and vertebral arteries at the skull base. With superselective contrast injection, the volume is reduced to 3 ml/s. Several circulation times (Fig. 105-2) are then measured frame by frame in the angiography console: nidal filling time (NFT), time from the first frame with nidal staining to the corresponding frame at maximum arterial enhancement; nidal time (NT), includes NFT up to the last frame outlining the arterial nidus; draining time (DT), from the time of the first or predominant draining vein image, when venous draining is multiple, to the time when the abnormal draining vein or veins are no longer seen; total circulation time (TCT), time elapsed from the first frame of the nidal filling time to the last draining frame; and early shunt time (EST), time from the first frame of nidal filling to the first frame of venous draining time.
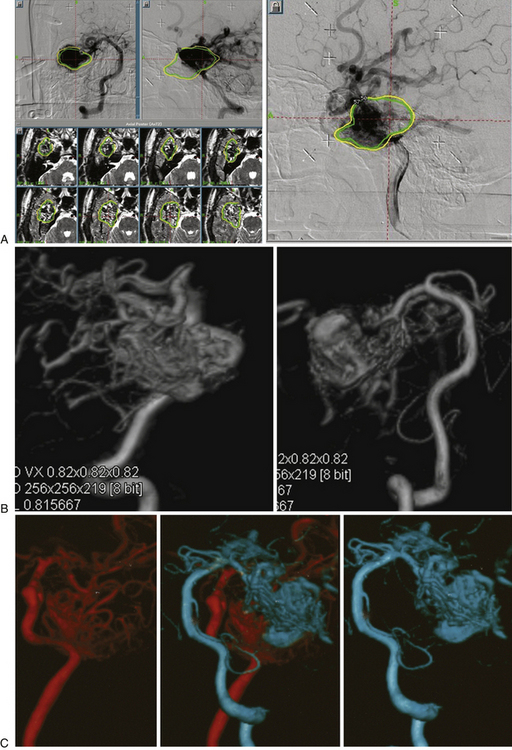
As the circulation times are studied and recorded, the various AVM components are defined, including the single or multiple compartments (Fig. 105-3), the type of venous drainage, and several flow patterns, depending on the circulation time compatible with the three types of circulation: I-A direct fistulae, I-B predominantly fistulous, II-A mixed fistulous-plexiform without stenosis of the draining vein, II-B mixed with draining vein stenosis, and III predominantly plexiform (Table 105-2).
Nidal circulation times (NTs) less than 2 seconds and a total circulation of less than 6 seconds correlate with the I-A and I-B fistulous flow patterns. Shunt (EST) times of less than 2 seconds and nidal filling times (NFTs) of 2 to 4 seconds and total circulation times of up to 8 seconds correlate with the mixed fistulous-plexiform flow pattern, and nidal times greater than 4 seconds and total circulation time greater than 9 seconds reflect predominantly plexiform flow. It is important to note that a patient with a II-B flow pattern with an early shunt time less than 1.5 seconds and a slow draining time greater than 7 seconds, resulting from stenosis of the predominant venous drainage, should be considered a candidate for embolization to reduce the risk of bleeding.
Our embolization protocol for radiosurgery depends on the volume of the AVM, the corresponding flow pattern, and the risk factors for bleeding. If the size of the pre-embolization target is less than 10 ml, the treatment volume should include all the original volume. If the pre-embolization volume is greater than 10 ml, the prescription dose is delivered to the residual volume in the critical zone. In terms of the flow pattern, it is advisable to use embolization to reduce the flow in patterns I and II-B. Endovascular therapy is also used to eliminate high-flow aneurysms or reduce volume in prospective staged protocols (Fig. 105-4).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree