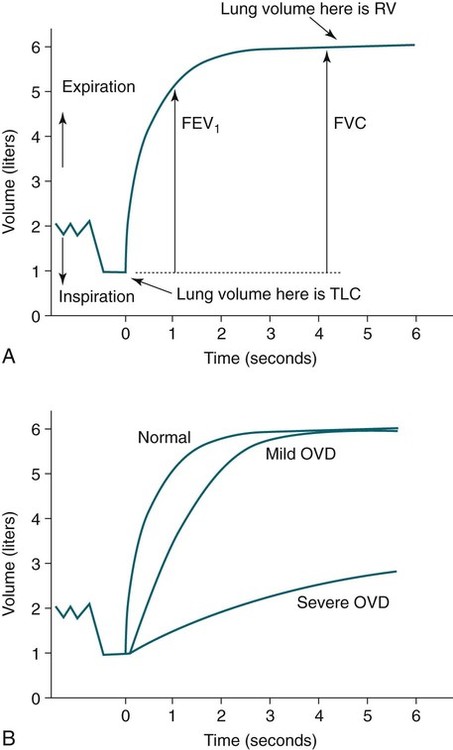
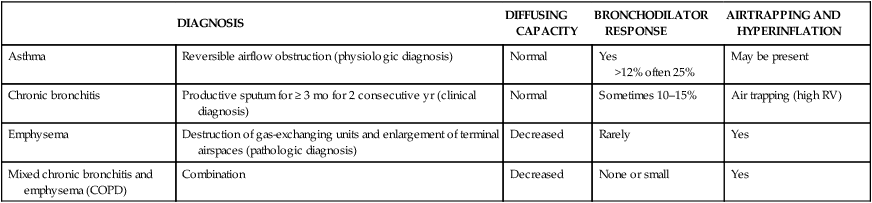
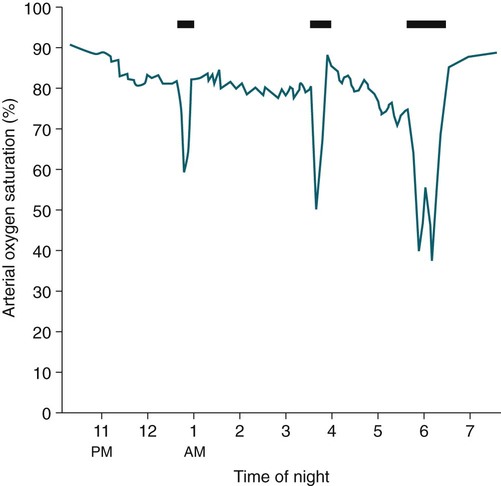
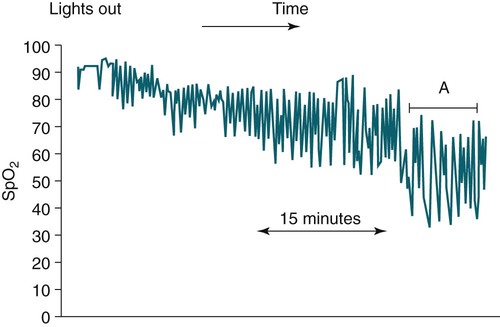
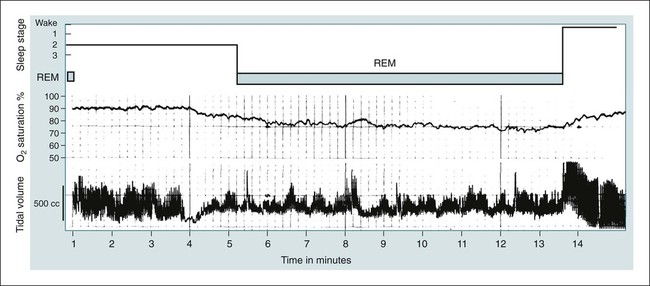
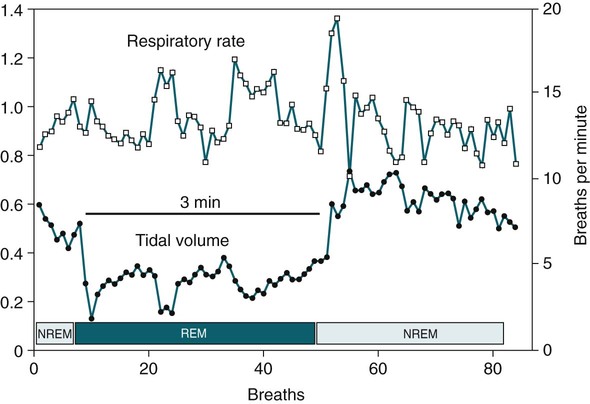
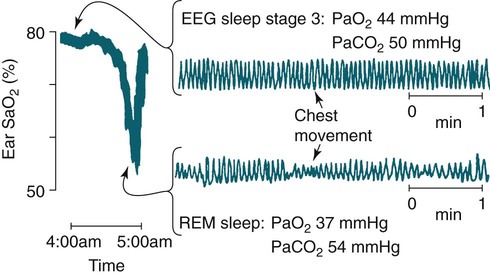
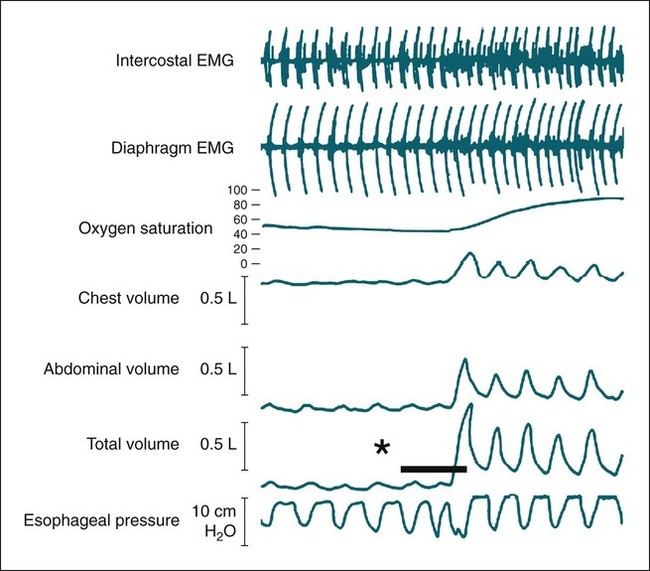
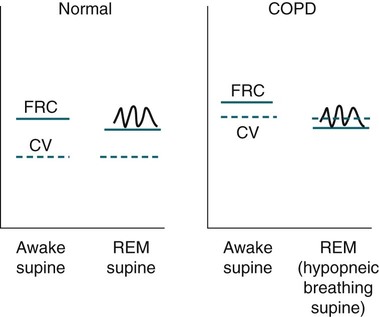
• Patients with COPD may experience NOD without discrete apneas and hypopneas. • In COPD, the NOD is worse during REM sleep. • NOD during REM sleep is often characterized by long periods of irregular and reduced tidal volume. • A saw-tooth pattern on a nocturnal oximetry tracing in a COPD patient suggests the possibility of coexisting sleep apnea. • If the awake SaO2 is low, even the normal fall in PO2 with sleep will result in greater desaturation (drop in SaO2) due to the initial position on the steep portion of the oxyhemoglobin dissociation curve. • Mechanisms of NOD in COPD patients include a low baseline SaO2 as well as hypopneic breathing during REM sleep characterized by a low tidal volume (hypoventilation) and a reduction in the FRC (greater • Nocturnal oxygen supplementation has not been proven to improve sleep quality in patients with COPD. • Long-term oxygen treatment (24 hr/day) improves survival in COPD patients with daytime hypoxemia (PaO2 ≤ 55 mm Hg, SaO2 ≤ 88% OR PaO2 55–59 mm Hg and evidence of cor pulmonale; see Box 22-2). • The benefit of nocturnal oxygen supplementation in patients with a daytime PaO2 ≥ 60 mm Hg is unproven. However, nocturnal supplemental oxygen is usually provided if significant nocturnal hypoxemia is documented (>5 min with SaO2 ≤ 88%; see Box 22-2). • Poor sleep quality in COPD patients is believed due to medication side effects, nocturnal cough, and dyspnea. • Bronchodilator therapy improves nocturnal gas exchange in COPD, but only a few studies have documented an improvement in sleep quality. If bronchodilators are used, medications with a long duration of action are preferred. • Benzodiazepine receptor agonists can be used with caution to treat insomnia in stable COPD patients without hypercapnic respiratory failure. A melatonin receptor agonist (ramelteon) was demonstrated not to worsen gas exchange and did improve sleep quality in one study. Sedating antidepressants are another option, but their efficacy as a hypnotic in COPD patients is unproven. • Patients with a combination of COPD and OSA (OLS) often require treatment with a combination of PAP and supplemental oxygen. Bronchodilator therapy may improve nocturnal gas exchange and smoking cessation should be encouraged. • BPAP may be better tolerated than CPAP in some patients with the combination of COPD and OSA. • Nocturnal oxygen therapy in hypercapnic COPD patients without OSA generally results in only mild increases in the PaCO2. A greater increase in nocturnal PaCO2 may occur in patients with a combination of OSA and COPD when supplemental oxygen is administered. • Studies suggest that OLS patients on long-term oxygen therapy have better outcomes if OSA is effectively treated with PAP. • Patients with nocturnal asthma experience a greater than normal fall in the FEV1 (morning dippers). This can be documented by peak flow measurements at bedtime and on awakening. • Frequent awakening from nocturnal asthma is one of the defining characteristics of moderate to severe asthma. The presence of nocturnal asthma symptoms means asthma is suboptimally controlled. • An inhaled corticosteroid is the first step in treating patients with nocturnal asthma. The next option is addition of a long-acting inhaled beta agonist. Obstructive ventilatory dysfunction (OVD) describes a pattern of pulmonary dysfunction characterized by a reduced ratio of the forced expiratory volume in 1 second (FEV1) to the forced vital capacity (FVC) on spirometry. In addition to the reduced FEV1/FVC ratio there is a reduction in flow manifested by a reduction in the FEV1 (Fig. 22–1).1–4 Patients with very mild OVD may have a normal FEV1 but a reduced FEV1/FVC ratio. For convenience, the lower limit of normal for the FEV1 is assumed to be 80% of predicted and the lower limit of normal for the FEV1/FVC ratio is assumed to be 0.70. However, using a slightly higher value of the FEV1/FVC ratio for the lower limit of normal is appropriate in younger patients. For example, using 90% of predicted as the lower limit of normal for the FEV1/FVC ratio may be more sensitive for identification of OVD. Patients with OVD have normal or increased lung volumes (residual volume [RV], function residual capacity [FRC], and total lung capacity [TLC]) (Fig. 22–2). The first lung volume to be affected is the RV. Most patients with OVD have an elevated RV due to airtrapping from small airway narrowing and closure. With more severe disease, the FRC and then the TLC may also be increased (hyperinflation). However, the relative increase in the RV is greater than any increase in the FRC or TLC. In moderate to severe OVD, the increase in RV is large enough (compared with any increase in the TLC) to cause a reduction in the FVC (see Fig. 22–2). The increase in the FRC and TLC is due to loss of lung elastic recoil (emphysema) or airway disease (asthma). The common OVD disorders are listed in Table 22–1. These include asthma (bronchospasm = reversible airway obstruction), chronic bronchitis (productive cough/sputum production usually with OVD), and emphysema (destruction of alveoli and increased size of terminal airspaces). Patients often have a mixture of these manifestations and are said to have chronic obstructive pulmonary disease (COPD). Patients with asthma may have normal pulmonary function between exacerbations that can occur after upper respiratory tract infections, exposure to allergens, or exercise. They typically demonstrate a large improvement in the FEV1 or FVC after inhaled bronchodilator. Asthmatics have bronchial hyperresponsiveness to methacholine challenge (>20% fall in FEV1 after inhaling low concentrations), allergen, or exercise challenge (>20% fall in FEV1 after exercise). A diagnosis of asthma can be made in a patient with normal pulmonary function by demonstration of bronchial hyperresponsiveness. Some asthma patients have persistent airflow obstruction and require inhaled or even oral corticosteroids for improvement. Patients with severe asthma can have hyperinflation and even hypercapnic respiratory failure. Patients with COPD and predominantly chronic bronchitis typically have recurrent exacerbations and tend to have lower arterial partial pressure of oxygen (PaO2) values earlier in the disease course. Some may develop hypercapnia and cor pulmonale (blue bloaters). Other OVD patients have predominantly emphysema with severe hyperinflation (due to loss of lung elastic recoil) and relative preservation of PaO2 levels until late in the disease course. They present with dyspnea and, because of the relatively spared PaO2, are called “pink puffers.” TABLE 22–1 Spectrum of Obstructive Ventilatory Dysfunction In OVD the increase in RV > increase in FRC > increase in TLC. COPD is the fourth leading cause of death in the United States.4 The major cause of COPD is cigarette smoking. Patients with COPD may die from respiratory failure or lung cancer. Although they typically present to physicians with complaints of bronchitis or dyspnea, they also frequently complain of poor-quality sleep. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has defined severity criteria (Table 22–2).4 In general, an FEV1 less than 30% to 40% of predicted or 1.0 L for a normal-sized individual indicates very severe disease. Daytime hypercapnia is usually associated with an FEV1 of 30% to 40% of predicted or less. Patients with the overlap syndrome (COPD + obstructive sleep apnea [OSA]) can develop hypercapnia with higher FEV1 values. TABLE 22–2 GOLD Criteria for Chronic Obstructive Pulmonary Disease Severity From Rabe KF, Hurd S, Anzueto A, et al: Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. Sleep in patients with COPD is often impaired in both duration and quality.5–7 Many patients also have significant hypercapnia and hypoxemia at night. Ten percent to 15% of patients may also have concomitant OSA that worsens nocturnal gas exchange (overlap syndrome). The typical pattern of nocturnal oxygen desaturation (NOD) in a patient with COPD is shown in Figure 22–3. The baseline sleeping arterial oxygen saturation (SaO2) falls 2% to 4% from the awake baseline with minor fluctuations until much larger drops are noted during rapid eye movement (REM; stage R) sleep. In contrast, a typical oximetry of a patient with the overlap syndrome is shown in Figure 22–4. There is a low baseline sleeping SaO2 and a saw-tooth pattern consistent with repeated discrete events. Whereas central sleep apnea could cause a saw-tooth pattern, this is most likely to represent OSA. Patients with COPD often experience exaggerations of normal sleep-related changes in ventilation. The most severe desaturation occurs during REM sleep. The major mechanisms of nocturnal arterial oxygen desaturation are listed in Box 22–1. The relative importance of hypoventilation and ventilation-perfusion ( During non–rapid eye movement (NREM) sleep, CO2 production falls but alveolar ventilation falls proportionately more and arterial pressure of carbon dioxide (PaCO2) increases slightly (Fig. 22–5). Recall that PaCO2 = constant × (CO2 production)/(alveolar ventilation); see Chapter 10. The fall in ventilation is due to a loss of the wakefulness stimulus to breathe, decreased ventilatory responses (chemosensitivity) to hypoxia and hypercapnia, and increased upper airway resistance.5,7 The increase in PaCO2 results in a mild decrease in the PaO2. However, because the awake PaO2 value is on the flat portion of the oxygen-hemoglobin saturation curve (Fig. 22–6), minimal drops in the SaO2 are noted (e.g., from 97% to 95%). The FRC may decrease slightly from wake to NREM sleep (Fig. 22–7).8,9 During REM sleep in normal individuals, ventilation is irregular and periods of reduced tidal volume occur, often during bursts of REMs. Skeletal muscle hypotonia reduces the contribution from the accessory respiratory muscles and respiration depends on the diaphragm.8,9 Two studies did not find a lower FRC during REM compared with NREM sleep in normal individuals (see Fig. 22–7).8,10 During REM sleep, there is typically a decrease in chest wall movement,9 likely due to chest wall hypotonia. These REM-associated physiologic changes result in a slight increase in PaCO2 and decreases in PaO2 during REM compared with NREM sleep. The slight fall in PaO2 results in minimal changes in the SaO2 due to the position of the oxygen-hemoglobin saturation curve (see Fig. 22–6). Koo and colleagues11 studied 15 patients with severe COPD (FEV1 of 0.96 L) with a mean daytime PaO2 greater than 60 mm Hg and found a mean decrease in PaO2 of 13.5 mm Hg and a mean increase in PaCO2 of 8.3 mm Hg during REM sleep (Fig. 22–8). Although patients with daytime hypercapnia or a low awake PaO2 or SaO2 are more likely to have worse gas exchange during sleep, up to 25% of patients with COPD exhibit REM-related hypoventilation and NOD, despite having a daytime PaO2 above 60 mm Hg.12 During supine wakefulness, patients with COPD often start with low-normal or slightly decreased SaO2 values. Therefore, even the normal fall in PaO2 with sleep will cause a greater decrease in the SaO2. Koo and colleagues11 documented a fall in PaO2 of approximately 8 mm Hg from wake to NREM sleep and an increase in PaCO2 of about 5 mm Hg. In patients with COPD, the onset of NREM sleep is associated with mild falls in the SaO2 (4–8%) and PaCO2 increases. If obstructive apneas or hypopneas are present, the degree of desaturation is worsened. Due to hyperinflation, the diaphragm is at a mechanical disadvantage and there is more dependence on the accessory muscles of respiration. In a study by Becker and coworkers13 of a group of hypercapnic patients with COPD, the minute ventilation fell by 16% from wake to NREM. During REM sleep, there are more profound periods of arterial oxygen desaturation compared with NREM sleep in patients with COPD. These episodes of desaturation are characterized by long periods of irregular breathing and reduced tidal volume (Figs. 22–9 and 22–10). In contrast to obstructive hypopnea in OSA patients, the onset and termination of these nonapneic periods of REM desaturation are less well defined. As noted previously, it is believed that REM-associated hypotonia reduces the contribution for the intercostal muscles so that ventilation depends on the diaphragm. Hyperinflation in patients with COPD reduces the effectiveness of the diaphragm. During the periods of REM-associated diaphragmatic inhibition (often during bursts of eye movements),13–15 the tidal volume and minute ventilation often decrease dramatically (see Figs. 22–9 and 22–10). The drop in alveolar ventilation is even larger than the change in minute ventilation due to an increase in the dead space–to–tidal volume ratio (see Chapter 10). In one study of a group of hypercapnic patients with COPD, the minute ventilation fell by 32% from wake to REM sleep (compared with 16% from wake to NREM).13 Studies have documented increases in PaCO2 during these episodes.11,15,16 Figure 22–10 illustrates a nonapneic period of REM-associated fall in tidal volume. Whereas hypoventilation definitely occurs during periods of nonapneic desaturation (also called hypopneic breathing), Catterall and associates16,17 directly measured arterial blood gases and found the PaO2 dropped more than the PaCO2 increased (Fig. 22–11). Therefore, increased Many patients with COPD have an increase in FRC in the upright position (hyperinflation). In the supine position, the FRC may be slightly lower during wakefulness. Ballard and coworkers10 found similar FRC values during wake, NREM, and REM sleep in patients with emphysema. However, in asthmatics, another study found that the FRC does decrease from wake to NREM and from NREM to REM (see Fig. 22–7).10 COPD patients with a significant component of airways disease could experience a drop in FRC during REM sleep. In a given patient with COPD, the FRC may also be affected by the degree of obesity. Furthermore, assigning a single FRC value to stage R sleep is problematic because breathing during REM sleep is very inhomogeneous. Hudgel and coworkers14 found no decrease in FRC during transition from stage N2 to stage R sleep in a group of five patients with COPD. However, during episodes of hypopneic breathing in REM sleep, there was a fall in FRC. The same study found that those COPD patients who desaturated during REM sleep had longer periods of hypopneic breathing (Fig. 22–12). Thus, it seems likely that the FRC during hypopneic breathing in COPD is lower than during NREM sleep. Whether the absolute value is below normal may vary between patients. A fall in the FRC during hypopneic breathing in COPD patients may have greater consequences than in normal individuals. In COPD, the closing volume (CV), the volume at which small airway closure is significant, is closer to the FRC than in normal individuals. During the hypopneic breathing of REM sleep, the FRC falls below the CV (Fig. 22–13). This means that during tidal breathing, more alveoli are either not ventilated or underventilated. This results in worsening of REM episodes in the early morning have greatest REM density and the greatest variation in ventilation even in normal individuals.19 These REM periods also are typically longer. In the early morning hours, there is greater lower airway resistance due to circadian changes in bronchomotor tone that are exaggerated in many patients with COPD.5,6 Ballard and colleagues18 found an increase in upper airway resistance but not lower airway resistance during the night in a group of patients with emphysema. Conversely, lower airway resistance does increase in asthmatics20 and likely in some COPD patients with a significant component of airway disease. These factors help explain why the most severe and longest REM-associated desaturation typically occurs in the early morning hours. Several studies have tried to find factors predicting more severe desaturation during sleep in COPD. As might be expected, a lower awake PaO2 and higher PaCO2 predict more dramatic changes in gas exchange during sleep. One study compared blue bloaters and pink puffers and found the former were more likely to desaturate during sleep (Table 22–3).21 Analysis of the Sleep Heart Health data suggested that the odds of having desaturation more than 5% of total sleep time was minimally increased until the FEV1/FVC ratio was less than 60% (Table 22–4).22 A plot of awake SaO2 versus minimum sleeping value is shown in Figure 22–14. Patients with lower awake SaO2 or PaO2 values tend to have lower values at night.23 However, there is considerable variability and oximetry or polysomnography (PSG) is needed to reliably exclude NOD. TABLE 22–3 Arterial Oxygen Saturation during Wake and Sleep—Variation with Chronic Obstructive Pulmonary Disease Type
Sleep and Obstructive Lung Disease
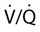 mismatch).
mismatch).
Obstructive Ventilatory Dysfunction
DIAGNOSIS
DIFFUSING CAPACITY
BRONCHODILATOR RESPONSE
AIRTRAPPING AND HYPERINFLATION
Asthma
Reversible airflow obstruction (physiologic diagnosis)
Normal
Yes
>12% often 25%
May be present
Chronic bronchitis
Productive sputum for ≥ 3 mo for 2 consecutive yr (clinical diagnosis)
Normal
Sometimes 10–15%
Air trapping (high RV)
Emphysema
Destruction of gas-exchanging units and enlargement of terminal airspaces (pathologic diagnosis)
Decreased
Rarely
Yes
Mixed chronic bronchitis and emphysema (COPD)
Combination
Decreased
None or small
Yes
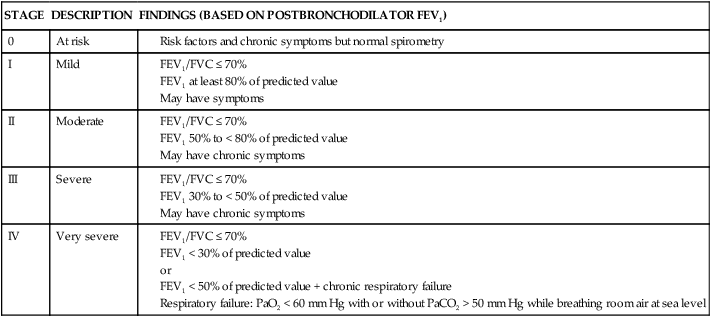
Chronic Obstructive Pulmonary Disease
STAGE
DESCRIPTION
FINDINGS (BASED ON POSTBRONCHODILATOR FEV1)
0
At risk
I
Mild
II
Moderate
III
Severe
IV
Very severe
Sleep in COPD
Etiology of Abnormal Nocturnal Gas Exchange
 ) mismatch is still debated.
) mismatch is still debated.
Normal Individuals
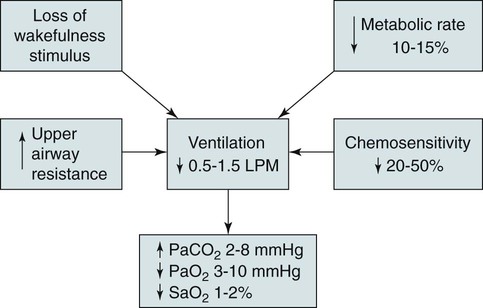
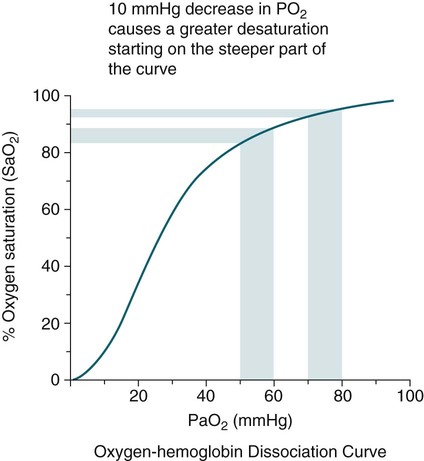
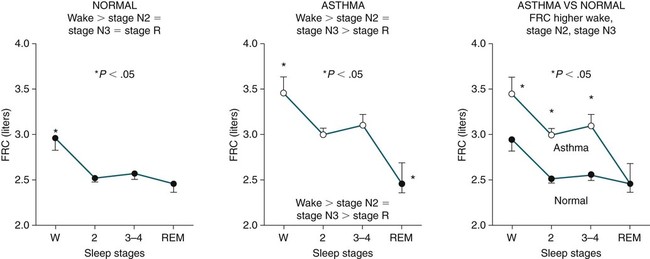
Sleep-Related Changes in Respiration in COPD
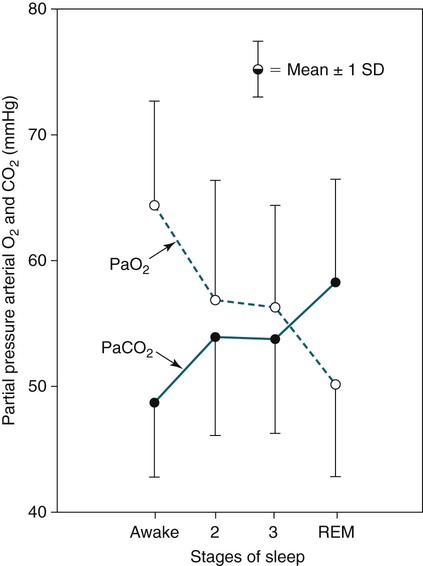
Non–Rapid Eye Movement
Rapid Eye Movement
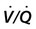 Mismatch
Mismatch
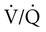 mismatch is believed to play a role in REM-associated desaturation (in addition to hypoventilation).15,16 However, as Catterall and associates16,17 and others have pointed out, the usual blood gas analysis of the relationship between PaO2 and PaCO2 depends on an assumption of steady state. This condition does not exist during the transient periods of hypopneic breathing. Because oxygen stores in the body are much smaller than carbon dioxide stores, a change in ventilation may affect PaO2 more than PaCO2.
mismatch is believed to play a role in REM-associated desaturation (in addition to hypoventilation).15,16 However, as Catterall and associates16,17 and others have pointed out, the usual blood gas analysis of the relationship between PaO2 and PaCO2 depends on an assumption of steady state. This condition does not exist during the transient periods of hypopneic breathing. Because oxygen stores in the body are much smaller than carbon dioxide stores, a change in ventilation may affect PaO2 more than PaCO2.
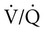 mismatch (already abnormal awake) and worsening hypoxemia. Hudgel and coworkers14 concluded that hypoventilation, and to a lesser extent
mismatch (already abnormal awake) and worsening hypoxemia. Hudgel and coworkers14 concluded that hypoventilation, and to a lesser extent 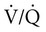 mismatch, causes REM-associated desaturation. Fletcher and associates15 found an increase in venous admixture during REM hypopnea episodes and concluded that
mismatch, causes REM-associated desaturation. Fletcher and associates15 found an increase in venous admixture during REM hypopnea episodes and concluded that 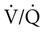 mismatch was a significant cause of hypoxemia. The relative roles of hypoventilation and
mismatch was a significant cause of hypoxemia. The relative roles of hypoventilation and 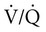 mismatch in inducing nocturnal desaturation during hypopneic breathing during REM sleep are still debated. The analysis is complicated by the non–steady-state condition of transient hypopneic breathing.
mismatch in inducing nocturnal desaturation during hypopneic breathing during REM sleep are still debated. The analysis is complicated by the non–steady-state condition of transient hypopneic breathing.
Time of Night and Circadian Variation in Lung Function
COPD Types and Respiration during Sleep
PINK PUFFER (%)
BLUE BLOATER (%)
SaO2 awake and sitting
93
88.4
SaO2 awake and supine
92.3
80.5
SaO2 sleep
92.3
77.3
Maximal fall in SaO2
4.7
29.5
Episodes of arterial oxygen desaturation
3.5
22.7
SaO2 < 80% (min)
0.08
120.0 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








