Spinal Arteriovenous Malformations
Etiology
Spinal vascular malformations form a diverse group of vascular entities, with disparate etiologies as well as presentation. Three main categories compose the majority of spinal vascular malformations: spinal arteriovenous malformations (sAVMs), spinal cavernous malformations, and spinal vascular tumors. Spinal AVMs have been classified in many ways, based on both their anatomy and pathogenesis; these classifications are discussed below.
Many classification systems for spinal arteriovenous malformations have been proposed, most of which are based on the nidus, the feeding artery, and anatomic location of the lesion. Spinal AVMs can generally be classified into four types (Fig. 20.1). Type I represents an arteriovenous fistula between a dural branch of the spinal ramus of a radicular artery and an intradural medullary vein. The fistula is usually located in the dural sleeve of a dorsal spinal nerve root. Type II represents an intramedullary arteriovenous malformation with a compact nidus within the substance of the spinal cord. Type III represents an extensive arteriovenous malformation, often extending into the vertebral body and paraspinal tissues. Type IV represents an intradural perimedullary arteriovenous fistula. This fistula is along the spinal cord and feeds from the spinal arteries, usually the anterior spinal artery.
Type I is the most common type of spinal AVM and accounts for approximately 60–80% of cases. These lesions are also synonymous with spinal dural arteriovenous fistula, the long dorsal AVM, angioma racemosum venosum, and the single-coiled vessel malformation. Type I spinal AVMs have been further classified into lesions with a single arterial feeder (Type I-A) or those with multiple arterial feeders (Type I-B). Failure to visualize all feeders in a Type I-B AVM may make it difficult to localize the draining vein and provide definitive treatment.
The exact etiology of these AVMs is unknown; they are believed to be acquired lesions that may occur either spontaneously or following trauma. They are commonly located in the dural sleeve of the lower thoracic and lumbosacral nerve roots. These malformations are, in fact, arteriovenous fistulas in the dural sheath of a spinal nerve. The fistula is supplied by a radicular artery or arteries, which drain into one and occasionally more veins located on the dorsal pial surface. As a result of the shunt, the coronal venous plexus, which normally drains the posterior two thirds of the spinal cord, becomes arterialized, and the resulting increase in venous pressure impairs spinal cord perfusion and venous drainage. These engorged veins may extend for several spinal levels; since they are valveless, they occasionally drain cranially and arterialize the posterior cranial fossa veins. Despite the high venous pressure, type I AVMs are relatively low-flow fistulas and rarely hemorrhage.
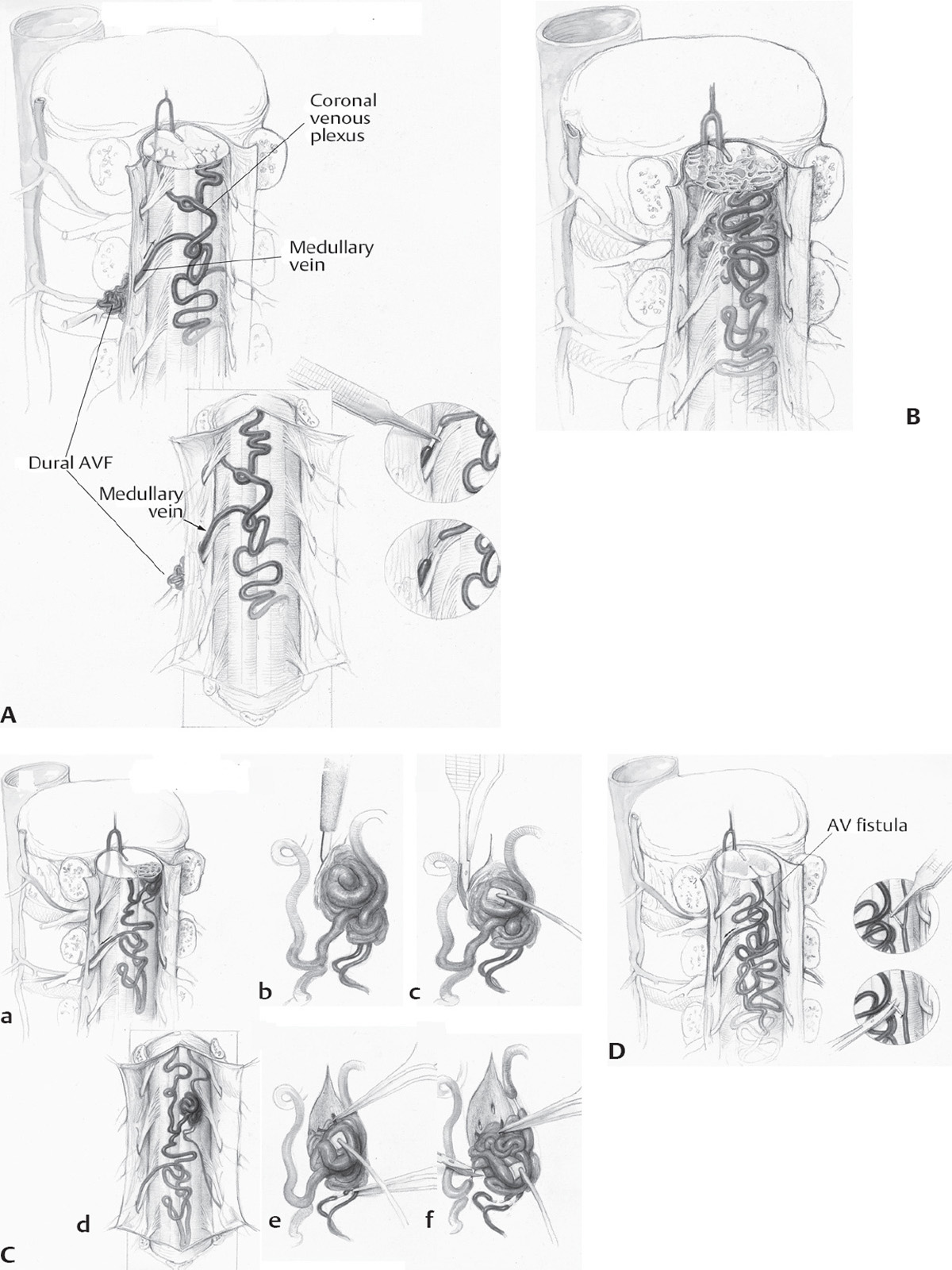
Fig. 20.1 (A) The vascular anatomy of a spinal dural arteriovenous fistula (AVF). The AVF is supplied by a dural artery and drained by a medullary vein. Arterial input into the valveless intradural venous system increases venous pressure within the coronal venous plexus and causes myelopathy. Treatment is to coagulate and divide the arterialized draining vein at the site of the AVF. (B) Vascular anatomy of a juvenile intramedullary arteriovenous malformation (AVM) of the spinal cord. The juvenile type of intramedullary AVM is fed by enlarged medullary arteries via dilated anterior and posterior spinal arteries. The nidus of the AVM is extensive, often filling the spinal canal, and contains neural tissue within the interstices of the vessels of the AVM. (Ca) Vascular anatomy of glomus intramedullary arteriovenous malformation (AVM). The nidus of the glomus type of intramedullary AVM is a tightly packed nidus of blood vessels confined to a limited segment of the spinal cord. (Cb) After separating the arachnoid from the AVM, a diamond knife is used to incise the pia at the edge of the nidus. (Cc) To obtain the necessary exposure, the pial incision is extended a few millimeters rostrally and caudally. (Cd) Surgical anatomy of spinal AVM. (Ce) The superficial feeding vessels are interrupted sharply after they have been coagulated. (Cf) Resection of the AVM, using a standard microsurgical technique. (D) Vascular anatomy of an intradural or perimedullary arteriovenous fistula (AVF). Medullary arteries provide the arterial supply, in this instance via a posterior spinal artery. Intradural AVFs often have associated arterial or venous aneurysms at the junction of the arterial and venous elements. Note the dilatation of the vein just distal to the AV shunt. The site of arterial to venous transition is identified, and after bipolar coagulation of a 4 to 6 mm segment of the distal portion of the artery(s) to the AVF, the AVF is interrupted on the distal portion of the arterial side of the fistula, beyond the last arterial branch to the spinal cord. In some instances, a small clip or ligature is required, but most feeders can be managed by bipolar coagulation and sharp interruption alone. If an aneurysm or varix is the site of convergence of the feeding vessels, it is excised. Many of these lesions are not as simple as the one illustrated here because these lesions can have more than one simple fistula in the same region of the pia, and the tortuosity and dilatation of the venous drainage often obscure the site of the AVF beneath a nest of blood vessels. (From Macdonald RL. Vascular neurosurgery. In: Neurosurgical Operative Atlas, 2nd ed. Thieme Medical Publishers; 2008.)
Type III AVMs (also called juvenile or metameric AVMs) are the least common type of spinal vascular malformation and appear to be congenital lesions. They are diffuse lesions that not only involve the spinal cord but may have significant extension into subdural and epidural spaces, as well as into the surrounding soft tissue and bone. In cases with involvement of all embryological layers of a metamere, the condition is known as Cobb’s syndrome. They may be associated with cutaneous angiomas and angiomatous phakomatoses, such as Klippel-Trenaunay-Weber syndrome and Rendu-Osler-Weber syndrome. The natural history is not well known; however, most patients do poorly.
Type-IV AVMs (also called perimedullary fistulas) appear to be acquired lesions that are most commonly located in the thoracolumbar region, occasionally are isolated to the thoracic levels, and rarely occur in the cervical region. These AVMs consist of a direct fistulous connection between the anterior or (less commonly) the posterior spinal artery and a spinal vein. As a result of the fistula, there may be massive aneurysmal venous dilatation, which may extend to the craniocervical junction and even into the posterior cranial fossa. The clinical manifestations may be caused by venous hypertension, arterial steal, or compression from dilated venous varicosities. In contrast to type I AVMs, which rarely hemorrhage due to the dural location of the fistula, perimedullary fistulas may occasionally present with subarachnoid hemorrhage.
Presentation of Spinal AVMs
Type I AVMs are most frequently found in males between the ages of 40 to 70 years. The most common clinical syndrome is progressive sensory and motor neurological deterioration. The mode of progression may be continuous, stepladder, or waxing and waning. A history of back or sciatic-like pain at the time of presentation may be seen in 40% of patients. Similarly, a history suggestive of neurogenic claudication with exacerbation of the pain and neurologic symptoms with exercise is not infrequent. Under these circumstances, it is likely that the venous hypertension, which is believed to be responsible for the progressive symptomatology, is exacerbated by foraminal narrowing that compresses exiting draining veins.
Type II AVMs usually present between the ages of 20 to 40 years. The clinical course may be an apoplectic neurological deficit or a gradually progressive change in sensory or motor function. Approximately one third of patients present with hemorrhage. Clinical manifestations may be caused by subarachnoid or intramedullary hemorrhage, arterial steal, compression by distended vessels and varices, or venous hypertension.
Type III AVMs tend to present in adolescents and young adults with apoplectic hemorrhage or progressive neurological decline. In addition to the findings seen with type II spinal AVMs, an MRI will demonstrate extraspinal extension of the vascular lesion. Type III AVMs are fed by multiple spinal and paraspinal arteries and drain diffusely.
Type IV AVMs commonly present in early to middle adulthood as a progressive ascending sensory motor disturbance associated with sphincter dysfunction. An MRI will demonstrate flow voids in the subarachnoid space without intra-axial components. Angiography remains the test of choice to demonstrate the fistulous point.
Imaging of Spinal AVMs (Fig. 20.2)
Imaging of spinal AVMs presents the same challenges as their intracranial counterparts. Most of these lesions involve high-flow hemodynamics, thereby necessitating imaging protocols that not only have high temporal resolution but also must have adequate spatial resolution to provide detailed images of the often small vessel calibers that are involved with these lesions.
CT and CTA have been used, but with limited success, even in the era of multi-detector CT scanners. The ill-defined dynamic capabilities, issues with bolus-timing, and limited spatial resolution of CT, in addition to the use of radiation and iodinated contrast agents, make CT a modality that can be used, but it is not the first choice for examining spinal AVMs. MRI and time-resolved MRA are more useful imaging modalities to locate the lesion within the spinal cord, to assess the presence of an intraparenchymal hemorrhage, intravascular thrombosis, a syrinx, and spinal cord edema or atrophy, and to determine the hemodynamic behavior of the lesion. Numerous studies have demonstrated that MR protocols make it feasible to visualize spinal AVMs and provide a useful modality, without the use of radiation or iodinated contrast agents. Despite this, catheter-based digital subtraction angiography (DSA) remains the gold standard, because it provides improved dynamic, high temporal resolution views of hemodynamic flow through the malformation and a more detailed understanding of its angioarchitecture, including the number and source of the arterial feeders arising from both the anterior and posterior spinal circulation, as well as information on the venous outflow system.
When imaging spinal AVMs, several characteristics should be noted during all the hemodynamic phases, whichever modality is used. The arterial phase should be characterized by the location and number of feeders. Often, several levels above and below the lesion should be examined so as to not miss occult feeding arteries. Further, other associated vascular lesions should be identified, such as perinidal aneurysms or venous varicosities. The nidal phase should be characterized by the extent of the lesion, location with respect to spinal level, and its anatomical characteristics, that is, intramedullary, intradural, or other. The venous phase should be characterized by the number and location of venous drainage. Often, there will be a single fistulous connection that is some distance away from the location(s) of the dilated venous complex. Careful angiographic determination of this fistulous point(s) is critical to appropriate therapy and resolution of arteriovenous fistulae.
Evidence-Based Medicine
Our understanding of the natural history of type I AVMs is largely deduced from a retrospective analysis of 60 patients reported by Aminoff and Logue in 1974.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








