24 The early diagnosis and management of metastatic spine tumors are essential to reducing pain, preserving or improving neurologic function, and improving quality of life. The three primary treatment modalities are radiation therapy (RT), surgery, and chemotherapy. Although these treatment modalities are all essential for treating metastatic spine tumors, no prospective trial has established the role of any modality as first-line therapy, and decision making for an individual patient is largely based on institutional experience and the individual preferences of the treating physician. At Memorial Sloan-Kettering Cancer Center (MSKCC), treatment decisions are based on a conceptual framework that evaluates oncologic, neurologic, and biomechanical considerations in the context of a patient’s medical comorbidities and extent of disease. Oncologic issues reflect the radiation- or chemosensitivity of a given tumor type. Neurologic issues include the presence and degree of radiculopathy, myelopathy, and radiographic epidural spinal cord compression. Biomechanical issues include the presence of mechanical pain, pattern of bone involvement, and presence of coronal (scoliosis) and sagittal (kyphosis) plane deformities. Evaluation of patients based on these concepts has provided a rationale to make treatment decisions. This chapter reviews the presentation, imaging considerations, and outcome studies that affect treatment decisions in the context of this conceptual framework. Back pain, the most common presenting symptom in patients with metastatic tumor to the bone or epidural space, often precedes the development of other neurologic symptoms by weeks or months. Back pain in a cancer patient is metastatic disease until otherwise proven. Two distinct types of back pain are encountered in patients with spine tumors: (1) biologic (a.k.a. tumor) and (2) mechanical.1 Biologic pain is predominantly nocturnal or early morning pain that generally improves with activity during the day. A variety of causes have been proposed including local release of cytokines, periosteal irritation, stimulation of intraosseous nerves, and increased pressure or mass effect from tumor tissue in the bone.2 Biologic pain generally responds to the administration of low-dose steroids (e.g., Decadron 12 mg qd). Definitive treatment of the underlying tumor with radiation or surgery often relieves this pain. The recurrence of biologic pain following treatment may be a harbinger of locally recurrent tumor. Mechanical pain results from a structural abnormality of the spine, such as a lytic destruction of the vertebral body resulting in instability. Clinical symptoms are important for establishing the diagnosis of instability, as radiographic criteria have not been firmly established for pathologic fractures. As opposed to biologic pain, patients present with pain that is worse with movement and dependent on the level of spinal involvement. Pathologic fractures of the atlantoaxial spine pain may present with severe pain in flexion-extension but virtually always have a rotational component. In the subaxial cervical spine and lumbar spine, pain is made worse with axial loads, such as sitting or standing. Patients with thoracic or thoracolumbar compression fractures often have severe pain when lying flat, presumably from extension of an unstable kyphosis. Mechanical pain does not typically respond to steroids but may be relieved with narcotics or an external orthosis, pending definitive surgical therapy. Patients with intractable mechanical pain are often considered strong candidates for operation. Neurologic signs and symptoms often begin with radiculopathy (nerve root symptoms) and are followed by myelopathy (spinal cord compression). Radiculopathy in the cervical or lumbar spine causes pain or weakness in the classic dermatomal distributions; however, thoracic radiculopathy occurs as a band-like pain at a segmental level. Some patients develop a mechanical radiculopathy resulting from instability and neuroforaminal compression by tumor. This pain occurs with movement and weight bearing, is relieved by lying down, and is typically accompanied by mechanical back pain. Myelopathy begins as hyperreflexia, a Babinski reflex, and clonus, but progresses to weakness (corticospinal tracts), proprioceptive sensory loss (dorsal columns), and loss of pain and temperature (spinothalamic tracts) below the level of the spinal cord compression. Autonomic dysfunction may result from spinal cord or cauda equina compression. Painless urinary retention suggests a neurologic cause.3 Isolated loss of bowel and bladder function, in the absence of motor or sensory symptoms, most often results from compression at the conus medullaris or from sacral tumor compressing the lower nerve roots. In lesions above the conus medullaris, autonomic dysfunction is frequently a late finding. Neurologic testing should not simply focus on sensorimotor function below the level of the lesion. This is important for several reasons. First, these patients often have multiple spine lesions, and it is important to determine exactly which ones are contributing to the patient’s symptoms. In addition, it is also important to adequately rule out other causes of symptoms, such as brain metastasis or peripheral neuropathy. Any patient with facial weakness or other cranial neuropathies requires cranial imaging prior to surgical intervention for metastatic spine disease. In addition, focal extremity weakness with normal or decreased reflexes may be caused by plexus or peripheral nerve compression, as is seen with brachial plexus metastases. Finally, adequate documentation of the patient’s neurologic status at the time of presentation is of utmost importance to judge either the response or deterioration during the course of treatment. The evaluation of spinal patients should include a pain assessment, quantitative neurologic score, general performance score, and quality-of-life assessment. Pain assessment can be most readily performed with a visual analog scale. The score can be converted to reflect mild (0 to 4), moderate (5 to 6), and severe (7 to 10) pain.4 The two most commonly used neurologic scales are the Frankel grading system and American Spinal Injury Association (ASIA) score (Table 24-1).5,6 Both assess motor function, with a score of “E” being normal and “A” being complete paralysis. Performance status reflects ambulation, medical comorbidities, and the extent of disease. A patient may have normal motor strength but be unable to ambulate due to loss of proprioception, severe mechanical pain, lower extremity fracture, poor nutritional status, or poor pulmonary function. We have used the Eastern Cooperative Oncology Group (ECOG) performance status as a functional assessment (Table 24-2).7 It is important to include both neurologic and performance status when reviewing outcomes in cancer patients. Several tools have been used to assess quality of life, but none has been developed to combine cancer issues with musculoskeletal concerns. A solution is to combine the Health-Related Quality of Life (HR-QOL) and Functional Assessment of Cancer Therapy Scale (FACT), developed by the American Academy of Orthopedic Modems, but this requires further validation.8
Staging, Classification, and Oncological Approaches for Metastatic Tumors Involving the Spine
 Clinical Presentation
Clinical Presentation
 Staging and Classification
Staging and Classification
| Grade | Description |
| A | Complete: no motor of sensory function below the level |
| B | Incomplete: sensory but no motor function |
| C | Incomplete: some motor function is preserved but a majority of the muscle groups below the lesion have a grade >3 |
| D | Incomplete: some motor function preserved but a majority of the muscle groups below the lesion have a grade >3 |
| E | Normal sensory and motor function |
| Grade | Description |
| 0 | Fully active, able to carry on all predisease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to perform light work |
| 2 | Ambulatory and capable of all self-care but unable to perform work activities (bedridden <50% of the time) |
| 3 | Capable of only limited self-care (bedridden >50% of the time) |
| 4 | Completely disabled, not capable of any self-care (bedridden 100% of the time) |
| Primary site | % of all spine metastases (n = 11.884) |
| Breast | 30.2 |
| Lung | 20.3 |
| Blood | 10.2 |
| Prostate | 9.6 |
| Urinary tract | 4.0 |
| Skin | 3.1 |
| Unknown primary | 2.9 |
| Colon | 1.6 |
| Other | 18.1 |
Metastatic tumors to the spine are classified based on numerous features including histology, location, and pattern of tumor. The most common spinal metastases are listed in Table 24-3. These tumors are further classified into relatively radioresistant and radiosensitive groups (Table 24-4), which impacts the decision to use radiation as the first-line therapy. Tumors may be further divided by the level and extent of spinal element involvement (e.g., vertebral body, posterior element, or circumferential) and degree of epidural compression. Thorough radiographic imaging is essential for treatment decisions.
Imaging
Advances in imaging have improved the sensitivity of detecting spinal metastases and the specificity of differentiating other processes that involve the spine. Magnetic resonance imaging (MRI) has revolutionized assessment of metastatic spine tumors, but many imaging modalities play a role in evaluating patients with metastatic spinal tumor including plain radiographs, bone scan, computed tomography (CT) scan, myelogram, and positron emission tomography (PET). The goal of imaging is to be 100% sensitive and specific in identifying tumor, giving precise anatomic detail, identifying distant metastases, and showing recurrent tumor following the placement of instrumentation. No single imaging modality accomplishes all of these goals, but understanding the advantages and disadvantages of different imaging modalities assists the clinician with patient screening and treatment planning.
| Sensitivity | Tumor histology |
| High | Lymphoma |
| Myeloma | |
| Intermediate | Breast |
| Prostate | |
| Low | Sarcoma |
| Renal cell | |
| Lung | |
| Colon |
Plain radiographs are often ordered as the first test to evaluate a patient with cancer who has new-onset back pain but are relatively poor screening tests for metastases. Visualization of a radiolucent defect on plain radiographs requires at least 50% destruction of the vertebral body. Additionally, metastatic tumor often infiltrates the bone marrow of the vertebral body without destroying the cortical bone. Compression and burst fractures are readily identified. Plain radiographs can identify sagittal (kyphosis) and coronal (scoliosis) plane deformities in a weight-bearing state, whereas spinal deformities imaged in a supine position by MRI or CT scans may be reduced and thus remain undetected. Dynamic flexion and extension films may be used to detect instability, although in our experience they are rarely necessary and may put the patient at risk for progressive spinal cord injury. Following surgery, plain films are the best imaging modality for assessing spinal alignment and structural integrity of the instrumentation.
Bone scans (technetium-99m-methylene diphosphonate) are more sensitive than plain radiographs for detecting spinal metastases (B9). The advantage of a bone scan is the ability to screen the entire skeleton with a single image. Patients with spinal tumors often have other bone involvement that may be causing symptoms or require intervention. For example, a patient with L2 vertebral body disease causing nerve root compression may have a concomitant, symptomatic tumor in the pelvis, hip, or femur; however, bone scans rely on an osteoblastic reaction or bone deposition to detect spinal metastases so that rapidly progressive, destructive tumors may not be detected.9,10 Bone scans are relatively insensitive for multiple myeloma and tumors confined to the bone marrow and have a low specificity for tumor.10 Fractures, degenerative disease, and benign disorders of the spine (Schmorl’s nodes, hemangioma) all may be positive. Additionally, paraspinal tumors that enter the epidural space through the neuralforamen can result in back pain and progressive neurologic symptoms that often are not detected on bone scan. In a review by Avrahami et al,11 21 of 40 patients (53%) with previously diagnosed tumor and symptoms referable to the spine had a negative CT and bone scan, but tumor was seen on MRI. Frank et al12 reviewed a series of 95 patients in which 28% had a negative bone scan with MRI showing tumor and a discordance rate between the two imaging modalities of 31%.
Until MRI became widely available, myelography and CT were the best diagnostic modalities for assessing acute spinal cord compression. The risks associated with myelography, including acute neurologic decompensation in patients with high-grade blocks, have diminished its role.13,14 CTs continue to be useful both for assessing the degree of bone destruction and for determining when bone rather than tumor is causing spinal cord compression. For patients who have had spinal reconstruction with placement of metallic instrumentation, including titanium, it may be difficult to obtain accurate images of the spinal canal with MRI.15,16 Myelography and postmyelogram CT images continue to be used for imaging these patients. Newer composite alloys, such as carbon fiber, will improve imaging with MRI, even further diminishing the role of myelograms.
Magnetic resonance imaging is the most sensitive and specific modality for imaging spinal metastases. Sagittal screening images of the entire spine reveal bone, epidural, and paraspinal tumor.17 The extent and degree of spinal cord compression can be readily appreciated, especially on T2-weighted images (Table 24-5). Hybrid scans of the brachial or lumbosacral plexus may reveal tumor in patients with extremity weakness that is not entirely related to spinal cord or root involvement. Leptomeningeal metastases are often well visualized but require the use of contrast agents (gadolinium-diethylenetriamine pentaacetic acid, Gd-DPTA).18
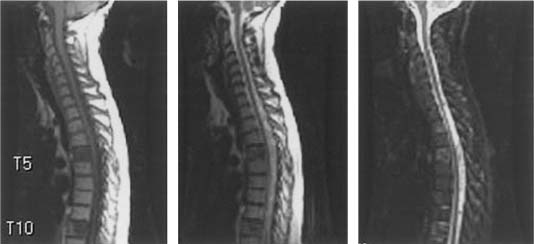
Common imaging sequences used to evaluate spinal metastases are T1-weighted and T2-weighted. Tumor on a T1-weighted image is hypointense relative to the normal marrow signal (Fig 24-1). The ports from prior spinal radiation can be discerned on T1-weighted images as hyperintense signal change and may assist in making acute therapeutic decisions when radiation port films are not available (Fig 24-2). Tumor is hyperintense relative to marrow on standard T2-weighted imaging and produces a myelogram effect with cerebrospinal fluid appearing hyperintense. Unfortunately, using the recently developed time-saving fast spin echo, T2 techniques may decrease tumor conspicuity. This decreased conspicuity can be compensated using short tau inversion recovery (STIR) techniques. STIR images show enhanced contrast between the lipid marrow (hypointense) and tumor (hyperintense) (Fig 24-1).19–21 They may be the most sensitive screening modality for tumor, but they give less anatomic detail than standard T1 or fast spin echo T2 images.22 Because of the high rate of multiple noncontiguous lesions, we suggest screening the entire spine with sagittal sequences followed by axial cuts through any areas of abnormality. Contrast administration is not routinely conducted unless there is a concern for leptomeningeal disease.
| Grade* | Description |
| 0 | No subarachnoid space compression |
| 1 | Subarachnoid space partially obliterated without spinal cord compression |
| 2 | Subarachnoid space partially obliterated with spinal cord compression |
| 3 | Subarachnoid space completely obliterated with cord compression |
Although MRI is an excellent screening tool for metastatic tumor spread to bone, differentiating tumor from osteomyelitis, osteoporotic compression fractures, and previously treated tumor may be difficult. The T1 and T2 signal characteristics are similar in all of these conditions. Osteomyelitis is more likely to cause changes in the end plate and disc space, whereas tumor rarely, if ever, involves the disc space. Based on these imaging characteristics, osteomyelitis can be differentiated from tumor with 97% accuracy.23 Unfortunately, patients with tumor may secondarily become infected, rendering the imaging patterns unreliable.24 We have encountered six patients over the past 4 years with documented tumor in the lumbar or thoracic spine that was treated with radiation therapy for radiosensitive tumors. The patients had progressive pain post-RT, and subsequent imaging showed vertebral body destruction but no specific MRI evidence of infection. All patients underwent surgical procedures for instability and presumed progression of tumor and were subsequently shown to have infection.
FIGURE 24-1 T1-, T2-weighted and STIR (left to right) sagittal mages resonance images (MRI) illustrating imaging characteristics of metastatic tumors in the T5 and T10 vertebral bodies.
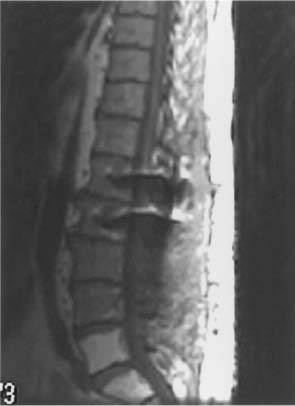
FIGURE 24-2 T1-weighted sagittal MRI showing hyperintensity of the L5 and S1 vertebral bodies as a result of prior radiation in this patient with metastatic sarcoma.
Osteoporotic compression fractures are extremely common in the cancer population and have been differentiated from pathologic fractures with 94% accuracy based on T1-weighted imaging characteristics.25 Osteoporotic fractures are more commonly thoracic, lack signal change, have band-like abnormality, and do not involve the pedicle or have contour abnormality. Pathologic fractures showed homogeneously decreased signal and convex vertebral contour involving the pedicles and lumbar location.
Oncologists often rely on imaging changes to determine the efficacy of treatment; however, response to RT or chemotherapy is difficult to assess in bone tumors because of the lack of signal change on MRI. On T1-weighted images, both treated and viable tumors appear hypointense relative to normal marrow signal. In a study of breast cancer patients, only 3% had a reduction in the volume or number of vertebral bodies involved on imaging.26 In a palliative situation, clinical response to therapy (resolution of tumor-related pain) may suffice despite the absence of radiographic change. Therapeutic decisions for some metastatic tumors (e.g., Ewing’s sarcoma, neuroblastoma, seminoma) rely on differentiating viable from necrotic tumor. MRI cannot reliably differentiate them.
We and other authors have begun to explore the use of 2-[F-18]-fluoro-2-deoxy-D-glucose (FDG)- PET for differentiating osteoporotic from pathologic compression fracture and to determine the viability of previously treated bone tumors.27 Osteoporotic compression fractures greater than 3 days from the onset of symptoms are hypometabolic with a standardized uptake value (SUV) of less than three, and most tumors have a SUV greater than three. Additionally, on T1-weighted images, bone edema may appear hypointense similar to tumor signal. FDG-PET has been useful in directing the biopsy to a specific hypermetabolic site in the vertebral body, increasing the chance of successfully making a diagnosis. Other radionuclide scans may be helpful for screening specific tumor types including iodine-131 scans for papillary thyroid cancer, metaiodobenzylguanidine (MIBG) scans for neuroblastoma, and somatostatin scans for neuroendocrine tumor.
Metabolic and Physiologic Issues
Cancer patients are prone to numerous metabolic and physiologic abnormalities either as the result of their disease process or as a side effect of previous treatments. Therefore, assessment for many of these abnormalities must be performed prior to considering treatment. Hypercalcemia occurs in about 10 to 20% of all cancer patients, with lung and breast tumors being the most common primaries.28 The pathophysiologic abnormalities that lead to this condition are believed to be secondary to the multifactorial effects of increased bone turnover and increased calcium reabsorption in the proximal renal tubules; however, immobilization and dehydration have also been shown to be contributing factors especially in patients with end-stage disease.28 These homeostatic abnormalities are now thought to be the result of secretion of a parathyroid-related protein as well as secretion of cytokines such as transforming growth factor β (TGF-β), interleukin 1 (IL 1), and tumor necrosis factor (TNF).29 Hypercalcemia is commonly treated with intravenous (IV) fluid rehydration and bisphosphonate administration; left untreated, it can result in cardiac or kidney dysfunction and even death in extreme cases.30
Coagulation abnormalities also occur commonly in this patient population. These can result from metastatic tumor spread to the liver or, more commonly, from the toxic side effects of chemotherapeutic agents. In addition, frequent blood transfusions that some of these patients receive may result in antiplatelet antibodies that may be resistant to replacement transfusions.
Diminished pulmonary reserve is another abnormality that is encountered quite commonly in patients with metastatic tumors. Patients undergoing thoracotomy for the treatment of lung cancer may be left with marginal reserve capacity. This is also seen as a result of multiple lung metastases, interstitial pulmonary fibrosis secondary to chemotherapy, pleural effusion, and the consequences of smoking. At our institution, all patients have a chest xray, and any patients with prior thoracotomy, any previously mentioned risk factor, or a history of dyspnea are evaluated with preoperative pulmonary function tests.
Cancer patients are also at an increased risk for developing deep venous thrombosis (DVT). The etiology is thought to be multifactorial and not simply a result of immobility. Many solid tumors release cytokines and other tissue factors that have procoagulant effects. We have found perioperative prophylaxis with pneumatic compression boots and subcutaneous heparin helpful in decreasing the rate of postoperative DVT, but not foolproof. When these are present preoperatively, they are managed with inferior vena cava filter placement. Postoperatively, DVTs are treated with either inferior vena cava filters or anticoagulation.
Many patients treated for spinal cord compression are also undergoing active chemotherapeutic treatment either for their primary disease or to control metastatic disease. A major concern is that many of these agents affect blood counts for several days after their administration. This may place patients at risk for neutropenia, anemia, or thrombocytopenia, all of which can have devastating consequences if not considered preoperatively.
Estimating Tumor Burden in Other Regions
The presence of distant metastases to extraspinal sites and active disease at the primary site are not contraindications to spine surgery, but recognizing the extent of disease is important for decision making. In patients with diffusely metastatic or rapidly progressive tumor, options such as radiation may be more appropriate; however, we often determine the appropriateness of surgical interventions based more on the patient’s overall medical condition as opposed to tumor load. Even in cases with limited life expectancy (3 to 6 months), decompression and stabilization may help preserve neurologic function, and thus quality of life, as well as palliate pain symptoms with an acceptable level of morbidity.
At MSKCC tumor staging is usually performed in conjunction with the primary oncologists, who have a better appreciation of the patient’s disease in terms of overall aggressiveness and degree of progression. This workup is typically done with radiographic studies including chest xray and CT scans of the chest, abdomen, and pelvis. In selected cases, MRI of the head, bone scans, or PET scans may be helpful. Certain tumors can also be assessed by following trends in serum markers such as prostate-specific antigen (PSA) or carcinoembryonic antigen (CEA).
Pain Control
The adequate control of cancer-related and postoperative pain can be very challenging in this population. A significant number of these patients may have chronic pain syndromes and require large doses of narcotics, typically in form of delayed release oral or transdermal preparations. This makes postoperative pain control difficult because of tolerance to these agents. At our institution, all patients receive narcotics via patient-controlled analgesia (PCA) (morphine, fentanyl, or Dilaudid) postoperatively for several days with frequent dosing modifications until adequate pain control is established. Following this, they are switched to equianalgesic doses of oral or transdermal medications. It is often helpful to obtain the input from pain management specialists for those patients with significant preoperative symptoms or high-dose requirements.
 Treatment
Treatment
Three treatment modalities are presently available for spinal metastases: (1) chemotherapy, (2) radiation therapy (RT), and (3) surgery. Chemotherapy can be divided into antitumor drugs and drugs that prevent or ameliorate the effects of tumor, such as steroids or bisphosphonates.
Antitumor Chemotherapy
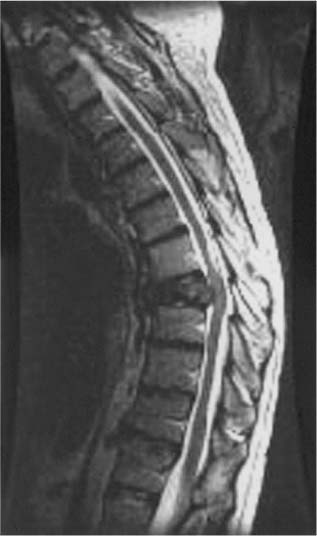
Antitumor chemotherapy currently plays a relatively limited role in the treatment of spinal metastases; however, antitumor chemotherapy has an important role in the treatment of chemosensitive tumors, such as neuroblastoma, Ewing’s sarcoma (primitive neuroectodermal tumor, PNET), osteogenic sarcoma, germ cell tumors, and lymphoma.31 At MSKCC, chemotherapy is often considered the primary treatment for patients with these tumors even in the presence of epidural compression (Fig 24-3). Surgery and RT may be used as adjuncts for residual radiographic tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree