Chapter 78 Surgical Management of Aneurysms of the Vertebral and Posterior Inferior Cerebellar Artery Complex
Aneurysms of the vertebral artery–posterior inferior cerebellar artery (VA-PICA) complex originate from any portion of the intradural VA up to the vertebrobasilar junction and from one of the five PICA segments. During the past decade, the treatment of these aneurysms became more sophisticated due to significant developments in diagnostic methods, improvements in microsurgical technique, further development of skull base surgery, better understanding of the microsurgical anatomy of the vertebrobasilar arterial territory, and dramatic advances in endovascular therapy. However, despite such continuous improvements, management of these complex lesions remains a challenging task. Several characteristic features distinguish VA and VA-PICA aneurysms from those of the anterior circulation: (1) they are relatively uncommon, occurring approximately one tenth as frequently as aneurysms in the anterior circulation1; (2) they show great variability in size, location, and morphology; the percentage of dissecting and fusiform aneurysms is much higher than in the other intracranial compartments; and (3) most of them are located deeply in the posterior fossa having a close relationship to the lower brain stem, the lower cranial nerves, and the cerebellum, making them difficult to access. The anatomic variability of the VA, the PICA, and the skull base around the jugular tubercle add further to the complexity of these lesions and increase the risk of their management. The infrequency of these lesions is the main reason why many neurosurgeons have only limited personal experience with the surgical treatment of VA-PICA aneurysms. With the advent of modern endovascular therapy, the number of lesions available for surgery, particularly the number of less complex VA and VA-PICA aneurysms, has further decreased, a situation that also raises problems in neurosurgical training. On the other hand, recent treatment strategies have gradually changed toward combined endovascular and surgical management, especially in the acute phase of severe subarachnoid hemorrhage (SAH) or in high-risk patients. It is obvious that only a limited number of specialized neurovascular centers can offer sufficient expertise for the safe management of this subgroup of vascular lesions.
Epidemiology
Aneurysms of the VA-PICA complex comprise 0.5% to 3% of all intracranial aneurysms.2–6 Approximately two thirds of these aneurysms are located at the bifurcation of the VAPICA junction, whereas distal PICA aneurysms account for approximately 0.3% to 1% of all aneurysms.6–8 In a recently published series of 24 patients, distal PICA aneurysms accounted for only 0.3% of all intracranial aneurysms and for 3.7% of the vertebrobasilar lesions; 74% were saccular, 7% were fusiform, and 19% were dissecting.7 Until 1992, only 140 patients harboring an aneurysm of the VA-PICA complex were reported in the literature9; of these, approximately 75% were VA or VA-PICA and 25% were distal PICA aneurysms. Multiple occurrence was occasionally reported.4,10–13 Although the incidence of aneurysms arising in association with arteriovenous malformations may be as high as 46%, the combination of a VA-PICA aneurysm and an arteriovenous malformation is rarely reported in the literature.14–16 With 85%, there is a clear predominance of female patients,4,5,17,18 even more notable in saccular aneurysms.19 Patients of virtually all ages may be affected, with an average age of 49.3 years.20
Aneurysm Characteristics
In contrast to intracranial aneurysms of other locations, only approximately 60% of VA-PICA aneurysms are saccular; approximately 30% are dissecting and 10% fusiform.9,21,22 In Yamaura’s18 series, there were 60% saccular, 27% dissecting, and 13% arteriosclerotic fusiform aneurysms; moreover, he found three giant lesions with a diameter exceeding 25 mm and two partially thrombosed saccular aneurysms. Drs. Drake and Peerless and colleagues have treated the world’s largest series of patients with vertebrobasilar aneurysms, comprising 1767 individual patients, of whom 217 (12.3%) harbored aneurysms of the VA-PICA complex.19,20,23 One hundred sixty-six lesions were saccular (76.5%), 25 were dissecting (11%), 18 were fusiform (8%), 4 were atherosclerotic (1.8%), 3 were associated with an arteriovenous malformation (1.3%), and 1 was traumatic (0.4%). The majority (70%) were small (<12 mm); 27 aneurysms (12%) were large (13 to 24 mm) and 40 (18%) were giant (>25 mm). Left-sided origin was more common (55%). Forty-three aneurysms (19%) were unruptured.19,23
Fusiform aneurysms appear as spindle-shaped dilatations, the vertebrobasilar trunk being the most frequent location for fusiform aneurysms.24 Due to a clear difference in natural history and optimal therapy, they must be clearly distinguished from dissecting aneurysms. More than two decades ago, nontraumatic dissecting aneurysms were considered to be extremely rare. However, improved neuroradiologic imaging techniques have demonstrated such lesions with increasing frequency, and during the past years, this type of aneurysm has received great attention in the pertinent literature.10,22,25 On arteriography, such aneurysms appear as a saccular or spindle-shaped vascular dilatation, occasionally combined with proximal stenosis. The classic arteriographic features of dissecting arteries include the double-lumen sign, retention of contrast medium, the pearl-and-string sign,26 and focal outpouching.22,27 Usually, they are not related to vascular branches of the VA. Such dissecting aneurysms of the VA may occur proximal as well as distal to the origin of the PICA, but occasionally they may involve the origin of the PICA as well.28 Yasui and colleagues29 believe that a fusiform VA aneurysm is one predisposing condition for a dissecting lesion. The sudden disruption of the internal elastic lamina is the primary mechanism underlying the development of dissecting aneurysms. The plane of dissection extends through the media, and most aneurysms have one entrance to this pseudolumen.30 Dissecting aneurysms of the VA often cause SAH by rupture of the adventitia and present a high risk of rebleeding.28,31,32 These lesions are not confined to the VA but may be observed on the distal PICA as well.7,33,34 Occasionally, a dissecting distal PICA aneurysm can develop as a traumatic lesion.35
Extreme dilations of the VA, termed “dolichoectasias,” occur less frequently and are usually difficult to treat. The involved artery is elongated and tortuous. On histologic examination, large defects within the muscular and elastic lamina can be detected and sometimes also extensive arteriosclerotic changes.30 Although dissecting aneurysms occur more frequently in male patients of younger age, dolichoectasias occur more frequently in the seventh decade.9,22,36,37
Historical Background
According to Hudgins and colleagues,4 the first case description of a saccular VA-PICA aneurysm was given by Cruveilhier in 1829. Rizzoli and Hayes38 were the first to surgically treat such an aneurysm in 1947 by interrupting the parent artery with two silver clips. Interestingly, the aneurysm was detected by these authors on a ventriculogram that showed a displaced fourth ventricle. Lewis and colleagues33 mentioned that the first case of an aneurysm arising from the distal segment of the PICA was reported in 1864 by Fernet and that the first surgical treatment of a peripheral PICA aneurysm is accredited to Olivecrona. In the 1950s and 1960s, vertebrobasilar aneurysms were associated with the highest mortality rate.39 Rizzoli and Hayes38 treated a peripheral PICA aneurysm with trapping in 1953. Uihlein and Hughes40 described in 1955 the nonsurgical treatment of 14 patients harboring a posterior fossa aneurysm; eight of these patients died after the aneurysm ruptured. After the introduction of routine vertebral angiography in patients with SAH, such aneurysms were detected with increasing frequency. In 1958, Desaussure and colleagues41 reported the successful surgical obliteration of two PICA aneurysms found on vertebral angiograms. Further improvements of neuroradiologic techniques after the introduction of transfemoral catheter and subtraction angiography as well as the routine use of microsurgical techniques in neurosurgery have dramatically improved the outcome of surgical procedures for treatment of vertebrobasilar aneurysms.42,43
Neuroradiologic Imaging
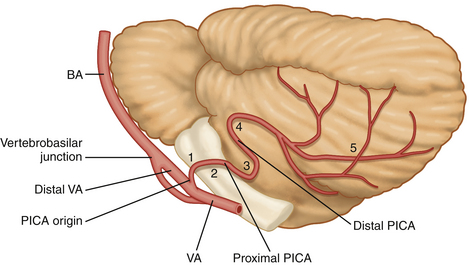
A meticulous preoperative neuroradiologic assessment is indispensable for successful treatment of VA and VA-PICA aneurysms. Neuroradiologic investigations should clarify the following features: (1) the exact location and origin of the aneurysm with respect to the VA and the various segments of the PICA (Fig. 78-1); (2) the size, shape, extent, and limits of the lesion to differentiate between saccular, fusiform, and dissecting aneurysms; (3) the orientation of the neck and the dome of the aneurysm; (4) the presence or absence of sufficient collateral circulation; (5) the patency of both VAs and the dominance of one of them, if present; (6) the presence or absence of multiple intracranial aneurysms or an associated arteriovenous malformation; (7) the precise relationship to the major surrounding anatomic structures as well as the degree of involvement of the brain stem and rootlets of the lower cranial nerves; and (8) the presence of hydrocephalus and/or intracerebellar/intraventricular hemorrhage.
Neuroradiologic studies include high-quality arterial digital angiography as well as various techniques of coronal, sagittal, and axial magnetic resonance imaging, and computed tomography (CT). Digital subtraction angiography may be complemented by rotational angiography with 3-dimensional rendering. Yonekawa and colleagues44 described three distances that can be measured on preoperative angiograms that are important predictors of the difficulty of operative access to VA-PICA aneurysms: the distance of the aneurysm from the midline, the distance from the most lateral point of the foramen magnum, and the distance from the clivus. According to these authors, optimal results are obtained when these distances are more than 5 to 10 mm, less than 10 to 21 mm, and less than 13 mm, respectively.
In a previous communication, we emphasized that high-resolution CT using a bone tissue algorithm is most useful to demonstrate the configuration of the skull base around the jugular foramen, in particular showing the size and shape of the jugular tubercle, the size of the posterior condylar canal, and the distance between the dural entrance of the vertebral artery and the hypoglossal canal or jugular tubercle.45 Special sections or 3-dimensional reconstructions may further add to the understanding of the individual skull base configuration or presence of bony anomalies. When performed with a bolus of contrast medium, this 3-dimensional CT image may demonstrate the relationship between the lesion, brain stem, and skull base, adding important information required for the planning of the procedure.45 Huynh-Le and colleagues46 have confirmed the utility of 3-dimensional CT angiography for the surgical management of VA-PICA aneurysms; this diagnostic technique was most valuable in demonstrating not only the exact site and shape of the aneurysm but also the relationships between parent vessel and malformation on the one side and the bony structures of the skull base on the other side. CT is important to demonstrate the SAH, the blood distribution within the basal cisterns, an associated hydrocephalus, and an intraventricular or intracerebellar hemorrhage. Intraventricular hemorrhage is present in as many as 82% of patients and associated hydrocephalus in approximately 75% of patients with ruptured aneurysms.5,47,48 Magnetic resonance imaging is particularly valuable in fusiform, dissecting, or partially thrombosed aneurysms.30,49 Magnetic resonance angiography can depict the aneurysm in relation to the brain stem, cerebellum, caudal cranial nerves, and skull base.
Clinical Presentation
The most frequent presenting symptom of patients with aneurysms of the VA-PICA complex is SAH. Rupture of these aneurysms occurs similarly to the rupture of aneurysms of the anterior circulation. However, the clinical consequences are far more disastrous. Although only a few patients experience intracerebellar hemorrhage or deficits of caudal cranial nerves, in some instances, prolonged coma, hemiparesis, or pulmonary embolism can occur.50,51 Aneurysm rupture occurs more frequently in lesions smaller than 12 mm. Large and giant aneurysms rarely rupture; they become symptomatic more frequently by their compressive effect on the lower brain stem or the caudal cranial nerves. Ischemic complications such as Wallenberg’s syndrome may occur in dissecting aneurysms due to occlusion of perforating arteries that supply the lateral aspect of the medulla. Patients with dolichoectatic VA and/or basilar artery may have ischemic stroke, brain stem compression, and occasionally hemifacial spasm or trigeminal neuralgia.52 In the series of Drake and Peerless53 of 221 patients, 178 had SAH (80.5%). Sixth nerve palsy, not lower cranial nerve paresis, was the most frequent preoperative cranial nerve dysfunction, as one might have expected. It was nearly always associated with SAH and recovered in 75% completely.
Management
The decision as to whether an aneurysm of the VA-PICA complex should be treated, as well as the timing and choice of treatment modality in case treatment appears indicated, depends on criteria such as the patient’s age, actual clinical condition, and neurologic status; progression or resolution of initial symptoms and signs; presence or absence of SAH; the interval between SAH and time of decision making; aneurysm characteristics; medical history; and the presence or absence of hydrocephalus and intraventricular or intracerebellar hemorrhage. Surgery, for instance, is preferable when a significant hematoma needs to be evacuated. For more than a decade, the concomitant availability of endovascular and microsurgical procedures has made possible a multimodal treatment of aneurysms of the VA-PICA complex.54–57 Particularly, the technologic achievements in neurovascular instrumentation (i.e., coil technology, intracranial stent technique) of the recent years led to a continuing challenge for interventional neuroradiologists, enabling them to treat even previously “untreatable” aneurysms with a high success rate.58–66 However, complications of the treatment such as brain stem infarction and hemorrhages are also reported for the interventional therapy of VA-PICA aneurysms.61,67,68 For some rare complex cases, a combined interventional and microsurgical therapy may constitute a reasonable solution.58
In some aneurysms such as proximal PICA lesions, even in cases in which endovascular coil occlusion of the aneurysms seems possible, the direct microsurgical inspection of the affected segment of the PICA and of perforating brain stem-supplying arteries may offer significant advantages compared with endovascular therapy.33 Considering the complexity and heterogeneity of VA and VA-PICA aneurysms, most lesions might require an individual case-by-case decision.
Surgical Approaches
To expose aneurysms of the VA-PICA complex surgically, a detailed analysis of aneurysm location, origin, extension, and orientation of the dome is necessary. Small saccular VA-PICA aneurysms may be exposed by a traditional suboccipital medial or lateral approach when they are located proximal to the rootlets of the lower cranial nerves. Aneurysms located more distally or even at the vertebrobasilar junction, as well as large or giant saccular or complex dissecting aneurysms, may require more extensive skull base approaches. Aneurysms of the third, fourth, and fifth PICA segments are best visualized via a suboccipital medial craniotomy. The patient is placed in either the sitting or prone position with the head flexed. The craniotomy includes the posterior rim of the foramen magnum. A number of lateral approaches are available to expose various aneurysms of the VA or the first two PICA segments.2,9,69–72 For exposure of some proximal VA aneurysms, a traditional retrosigmoid approach may be sufficient.73 However, several authors have underscored the necessity of extending the exposure more laterally.69 The retrolabyrinthine transsigmoidal approach was described by Giannotta and Maceri74 in 1988 and used to expose distal VA-PICA aneurysms or those of the vertebrobasilar junction.
It is a complex and more time-consuming skull base approach because large portions of the petrous bone must be drilled away and the ipsilateral sigmoid sinus is ligated, provided the contralateral sinus is intact. This approach, however, is rarely described in the literature for treatment of aneurysms of the VA-PICA complex. The transcondylar approach and several variations have been widely used by several authors in this context.33,75–79 A detailed description of the technique as we use it is given later in this chapter. Aneurysms that are not suitable for either surgical clipping or endovascular procedure may require other surgical techniques such as coating,5,80 external (surgical) trapping,4 or one of the various procedures of revascularization.
Saccular Aneurysms
Hernesniemi81 mentioned that most saccular nongiant aneurysms of the VA-PICA complex can be clipped. Today, most authors prefer an early treatment of these aneurysms after rupture, including distal PICA aneurysms,33 and also when they are associated with an arteriovenous malformation.15,82 The decision as to whether surgical clipping or endovascular coil occlusion is preferable should be made by an experienced neurovascular team. In the series of Horiuchi and colleagues7 comprising 27 PICA aneurysms in 24 patients, 22 lesions were clipped (81%), 2 (7%) were wrapped, 1 (4%) was proximally ligated with occipital artery-PICA bypass, and only 1 (4%) was occluded endovascularly with Guglielmi detachable (GD) coils.
Dissecting Aneurysms
With modern neuroimaging techniques, the diagnosis of dissecting aneurysms is now more precise than in the past.49,83 Although a benign course has occasionally been documented,84 the necessity of early treatment of dissecting aneurysms of the VA has been emphasized by many authors. An SAH from a dissecting aneurysm is considered a neurosurgical emergency because of a high incidence of rebleeding and a high mortality rate at the time of recurrent bleeding.22,30,31,55 Conversely, the natural history of non-SAH cases is relatively benign and therefore the treatment remains controversial.85–87 Despite early satisfactory results with a proximal occlusion of the VA,88 it is now well recognized that proximal occlusion of the affected VA by clip placement or endovascular procedure may not be sufficient.28 The primary goal of therapy is thus complete exclusion of the dissecting aneurysm from the circulation to avoid progression of the vascular dissection or a distal embolism with ischemic complications.28 In most cases, clip occlusion is impossible and wrapping alone may not be efficient.83 Test occlusion of the vertebral artery is performed, and thereafter either surgical or endovascular occlusion (internal trapping) is recommended.54 According to Hamada and colleagues,28 when occlusion of the VA is chosen as treatment option, patients should undergo a 20-minute occlusion tolerance test using a nondetachable balloon. Yoshimoto and Wakai87 have questioned the necessity of this balloon test because they doubt its reliability; these authors believe that definitive unilateral VA occlusion can be performed safely unless the contralateral VA is hypoplastic. Kitanaka and colleagues89 have mentioned that the choice of surgical technique depends on the location of the dissecting aneurysm in relation to the origin of the PICA. The VA can usually be occluded distal to the PICA origin, whereas a proximal occlusion of the VA may cause a secondary thrombosis of the PICA. A number of revascularization techniques have therefore been applied to occlude the VA with a consecutive reanastomosis of the PICA: PICA-PICA bypass at the level of the caudal loop,90–92 occipital artery-PICA,93–98 VA-PICA anastomosis with the superficial temporal artery or radial artery,99–101 and VA-PICA transposition.102
Giant Aneurysms
Despite the rare occurrence of giant aneurysms of the posterior circulation, these lesions have received much attention in the literature.11,103–106
The reasons for their frequent description are special characteristics of these lesions. The therapy of giant aneurysms is far more difficult that of those in the anterior circulation. Ausman and colleagues93 have recommended surgical clip occlusion in cooperation with cardiosurgeons under hypothermia and circulatory arrest.107 This method, however, has a specific morbidity and is associated with a certain mortality. The optimal therapy of giant aneurysms is the direct clip occlusion, which still bears a high risk for the patient. For this reason, the parent artery (VA) may be deliberately sacrificed or endovascularly occluded,108 the same technique as that used for dissecting or fusiform aneurysms. According to Inamasu and colleagues109 who treated six patients with giant VA and four patients with VA-PICA aneurysms surgically, more favorable results were obtained with surgical or endovascular rather than with conservative therapy.
The Authors’ Series
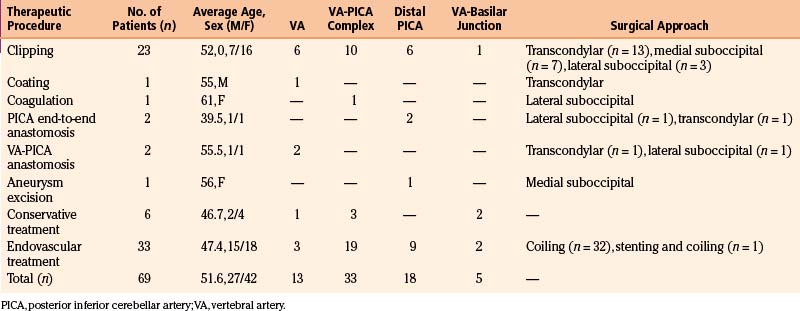
A total of 69 patients with aneurysms of the VA-PICA complex were treated in the period 1992 to 2004. Thirty patients were treated surgically, 33 endovascularly and 6 conservatively. Patient data are summarized in Table 78-1.
Surgical Anatomy
The origin of the PICA at the VA varies from extradurally, below the foramen magnum, to the vertebrobasilar junction. The PICA arises from the posterior or lateral surfaces of the VA more often than from the anterior or medial surfaces.110 The PICA is the artery with the most complex relationship to the cranial nerves of any artery.111 By definition, the PICA originates from the VA. Although rarely encountered (5%), one must bear in mind the possibility of an extradural origin of the PICA and of an extracranial (intradural) site of distal PICA aneurysms.110,112 In a few cases, the VA and the PICA are missing. If the PICA is present, it is the largest branch of the VA.110 Lister and colleagues113 have divided the artery into five segments based on its relationship to the medulla and the cerebellum. These five segments are the anterior medullary, the lateral medullary, the tonsillomedullary (includes the caudal loop), the telovelotonsillar (includes the cranial loop), and the cortical.113 Each segment sometimes includes more than one trunk. The PICA is closely related to the cerebellomedullary fissure, the inferior half of the ventricular roof, the inferior peduncle, and the suboccipital surface.110 The PICA supplies perforating branches to the medullar, choroidal arteries and cortical arteries. Perforating arteries arise from the medullary segment terminating into the brain stem.110 Recently, Marinkovic and colleagues114 gave a detailed description of the perforating branches of the VA providing valuable information for those performing aneurysm surgery in this region.
The upper portion of the sternocleidomastoid muscle and its posterior border indicate the region for the skin incision and further muscular opening. The most important muscles of the deep layer are the rectus capitis posterior major and minor muscles and the superior and inferior oblique muscles. The area between the medial rim of the superior oblique muscle, the upper rim of the inferior oblique muscle, and the lateral rim of the rectus capitis posterior major muscle, called the suboccipital triangle, contains the posterior rim of the atlantal arch, the horizontal portion of the vertebral artery, and the C1 root between these two structures. The posterior atlantal arch also serves as an important landmark for early localization of the VA during the stage of muscular dissection. Once this structure has been identified by palpation, the artery can readily be exposed in the sulcus dorsal and medial to the lateral atlantal mass. A C1 hemilaminectomy may not always be necessary but can be helpful when mobilization of the VA is required. The posterior edge of the occipital condyle is located just lateral to the dural entrance of the VA. To better expose the proximal intradural VA, this portion of the occipital condyle must be drilled away.
The diameter of the posterior condylar canal that contains the posterior condylar emissary vein varies widely. This canal is a very important landmark because it opens into the posteromedial margin of the jugular foramen and indicates the direction of bony drilling to expose the jugular tubercle. The jugular tubercle is a rounded prominence located at the junction between the basal part (clivus) and the condylar part of the occipital bone (jugular process). The jugular tubercle is one of the most important landmarks being surrounded by a number of vital neurovascular structures that must be exposed partially or totally.45,77 Medially and superiorly, the jugular tubercle is overcrossed by the cranial nerves IX, X, and XI intradurally. Laterally, the jugular tubercle has a close relationship with the jugular bulb, reaching the medial wall of the jugular foramen. Inferiorly, there is the hypoglossal canal containing the hypoglossal nerve surrounded by its venous plexus. Seeger115 was the first to document the necessity of resecting the jugular tubercle for an adequate visualization of near-midline aneurysms of the VA-PICA complex, later underscored by Perneczky.116
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree