Chapter 83 Surgical Management of Cerebral Arteriovenous Malformations
History
AVM neurosurgery is a relatively recent innovation. Even though there have long been reports about the surgical management of this kind of lesion, only from 1960 onward has the veritable surgery become a reality. In the 1960s, endovascular treatments started to be performed as a way to assist neurosurgeons in the resection of such lesions. Since the first description of embolization by Luessenhop and Spence in 1960,1 endovascular procedures have been improved, enabling a dramatic change in the direction of AVM treatments. In that same decade, with the appearance of the operating microscope, surgery took a 180-degree turn in everything regarding neurosurgery, including neurovascular surgery.1 Permanent laboratory practice in microsurgery, better and continued training in neuroanatomy, and constant development of surgical instruments for microsurgery, together with the supraspecialization in vascular neurosurgery, have led neurosurgeons to acquire more practice in the resection of such lesions, with a consequent decrease in morbidity and mortality. Eventually, radiosurgery came up and started to become popular as an adjuvant treatment—or as the only treatment for some cerebral AVMs—in the 1980s.
Classification
As AVMs became known, particularly their characteristics and the difficulties they posed in each case, they started to be classified according to different factors. Such classifications sought and still seek to help neurosurgeons decide whether to treat a cerebral AVM and what therapeutic procedures would be most appropriate for each case.2
In 1977, Luessenhop and Gennarelli proposed a classification based on the arterial pedicles that supplied AVMs.1,17 This classification did not take into account the draining veins or whether AVMs were located in eloquent areas. In 1984, Luessenhop, with Rosa’s collaboration, published a grading scale based on AVM size.4,18 Even though it was an easily applicable classification, it was not practical for surgery because it did not consider the venous drainage or whether it was in an eloquent area. Sugita also classified AVMs located at the sylvian fissure, taking into account whether they were directly in the fissure, lateral, medial, or deep seated.3 In 1986, Shi and Chen published a classification that took into account size, location, depth, the feeding arteries, and the venous drainage.4,18 It was a complex classification, one difficult to remember due to the various combinations therein (since it had seven grades), and the determination of eloquent or noneloquent areas was imprecise. But in that same year Spetzler and Martin’s classification was published, which took into account size, location, and venous drainage.4 This classification turned out to be the most practical one, and its diffusion and acceptance worldwide were fast and unanimous. As time went by, and with the application of this classification, it became evident that AVMs in grade III presented different difficulties in treatment and prognosis. This was motivated by the variability of the lesions included in this grade: for example, it included a small AVM with deep venous drainage located in an eloquent area, such as the brain stem, as well as a 6-cm-wide cortical lesion with superficial venous drainage located in a noneloquent area. This range led several authors to propose modifications to this grade of the classification. The most accepted ones are grades IIIA and IIIB, regarding location.3–6
Even though modifications are still being proposed, the Spetzler-Martin classification is the most commonly used at present, and treatment criteria are mostly based on it. Regardless, with the existing research possibilities on brain function, it is becoming increasingly difficult to speak of eloquent and noneloquent areas. In one way or another, practically the whole brain is “eloquent.”
Based on my experience, as well as on the work that has been carried out in Uruguay for year, and the concepts given by Borovich in the 1980s,6 I would add to the Spetzler-Martin classification the arterial territories involved in AVMs. They are grouped according to whether one, two, or three vascular territories are involved. Most AVMs are included in the groups for one or two vascular territories, which correspond, to a great extent, to grades I to III in the Spetzler-Martin classification.6
Treatment
In a publication about the management of AVMs in 1979, almost 20 years after the beginning of the AVM era, Drake proposed five options neurosurgeons could use when dealing with such a lesion7:
• Expectant behavior (which certainly refers to aggressive treatments, not to treatment symptoms, e.g., epilepsy)
After complete surgical resection of an AVM, with no evidence of remaining lesions in the angiographic control, the patient can be deemed cured. Although this happens in the vast majority of the cases, AVM relapses have been reported.8 It is my opinion that in such cases the angiographic subtraction control study failed to reveal small pathologic pedicles, from which the lesion subsequently reproduced.
Better knowledge of the natural history of AVMs, their flow, the pressure at the nidus, and AVM functionality, as well as that of the surrounding brain, has permitted substantial improvement in the prognosis of such lesions in the last two decades. This has been achieved through the use of dynamic studies, such as supraselective digital angiographies, digital subtraction angiography combined with color scaling, diffusion and perfusion magnetic resonance imaging with flow study, magnetic resonance imaging tractography, magnetoencephalography, and positron emission tomography. All these techniques allow better knowledge of the malformation, its behavior, and the characteristics of the surrounding brain, thus enabling the surgical team to plan the best treatment or combination of treatments for each case.9–16
Arteriovenous Malformations
Vascular malformations of the brain (excluding aneurysms) constitute a heterogeneous group of lesions that includes AVMs, pial malformations, cavernous angiomas, venous malformations, dural fistulae, and telangiectasias. Therefore, AVMs widely dominate the spectrum of lesions.17
Cerebral AVMs are lesions of likely congenital origin or predisposition constituted by a conglomerate of abnormal vessels (both arterial and venous) of variable size and number. There is no intermediate capillary network; there is no parenchyma among vessels. Usually, AVMs are surrounded by a thin layer of nonfunctioning brain tissue (reactional gliosis).6,17
Independent of their classification, AVMs can be divided into one of two categories, superficial (also called “of the convexity,” “cortical,” or “corticosubcortical”) or deep, when planning for their treatment.6,18 Given the variability of these lesions, in both their anatomy and their physiology, there is no universal consensus about how to treat them, even those AVMs that a priori could be similar. Thus, there have long been attempts to establish standards and guidelines for the treatment of AVMs. The problem lies in rapid changes to the proposed guidelines arising from development in diagnostic techniques and knowledge about AVMs and their treatments.
Regardless, the guidelines are recommendations formulated by groups of professionals dedicated to a particular pathology and must be taken as a base for the treatment of AVMs. They are flexible and can be modified according to the results of the different possible treatments and to the appearance of new therapeutics.19,20
Through its chapter on vascular neurosurgery, the Latin American Federation of Neurosurgical Societies in 2003 formulated the first recommendations for the management of AVMs.17 In 2009, I led a group of neurosurgeons dedicated to vascular surgery who published an updated version of those recommendations under the (translated) title “Recommendations for the Management of Brain Arteriovenous Malformations.”17 This publication aims to offer the specialist a guide for managing such lesions, but by no means is strict compliance necessary.17,19
In the last decade, several authors have published guides with the same objective (e.g., Vazquez and Larrea in 2000,21 Ogilvy et al. in 2001,19 and Starke et al. in 200922). These guides do not substantially differ in their recommendations, proposing surgery for grades I and II, combination of treatment for grade III, and conservative treatment for grades IV and V. Starke et al. conclude that surgery is the ideal and curative treatment. They advocate surgery, endovascular surgery, radiosurgery, or combination therapies depending on various factors: angioarchitecture of the AVM, topography, experience of the surgical team, and so on.19–22
AVMs are lesions whose incidence ranges between 1.4% and 4.3% of the population.23,24 The percentage of symptomatic AVMs is unknown, but it has been proved that they are “dynamic” lesions.23,24 In other words, a cerebral AVM diagnosed but not treated can show in a high percentage of the cases, under a periodic follow-up, changes in its anatomy, size, and symptomatology.23,24 Hence, the conservative (do nothing) treatment should not be considered in low-grade malformations or in high-grade AVMs that have shown important clinical aggressiveness, such as repeated bleeding and epilepsy that is difficult to control.
When a treatment for an AVM is proposed—which should be a curative one—it should have a lower morbidity and mortality than those accorded by the natural history of that particular malformation. The treatment or treatments to be carried out in each case issue from the discussion of a multidisciplinary group formed by neurosurgeons, neurointerventionists, neurophysiologists, neurointensivists, neuroanesthesiologists, and neurologists. With all the necessary studies that enable understanding of an AVM (computed tomography, magnetic resonance with functional components, digital angiography, etc.), the team can then decide whether to treat the lesion. If treatment is chosen, one or a combination of the previously mentioned options should be chosen. Every time the team decides to treat an AVM, a curative treatment should be sought, which means the elimination of the lesion. If the treatment was meant to be curative but a remnant of the lesion remained, the treatment is considered a failure. Frequently, palliative treatments are considered, for instance, when dealing with grade V AVMs with recurrent bleeding. In such cases, we know that the possibility of curing the patient is low, but a palliative treatment may significantly reduce the possibility of future bleeding.25–28
When and How to Treat a Cerebral AVM
Cerebral AVMs manifest clinically through bleeding in 50% of the cases, followed by epilepsy. When an AVM has bled, it must be treated. When we face epilepsy refractory to the medical treatment, it must also be treated. When we face an AVM with no bleeding or significant clinical manifestations but with studies that show it presents intranidal aneurysms, stenosis of the afferent vessels, or significant venous stasis, it also must be treated. If AVMs are small and deep seated, they also must be treated, since it is accepted that they have a greater chance of developing complications. Regardless, studies have not been able to demonstrate—based on levels of evidence—a clear correlation between the characteristics of the lesion and its chances of bleeding.26,27,29,30
Following the analysis of malformations according to the Spetzler-Martin grading scale, the following guidelines could be established.17
Grade I and II AVMs
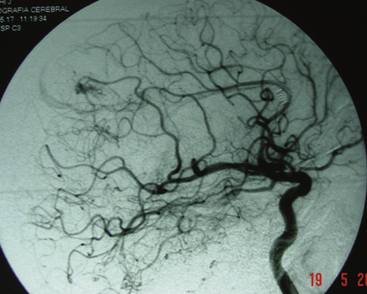
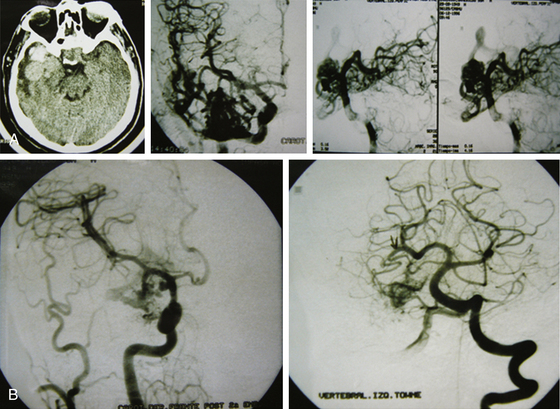
The endovascular option would be, for this group, in the third place. Even though they should not represent any difficulty for the endovascular surgeon, the angiographic disappearance of the lesions does not ensure they have been cured. Small, nonvisible pedicles may exist that may make the nidus reappear.2,5,6–19,21–23,25,31–37 Even though previous papers mentioned total and definitive occlusion with histoacryl glue,38 recent publications about treatment with Onyx reinforce that grade I AVMs can be cured with endovascular treatment (Fig. 83-1).39,40
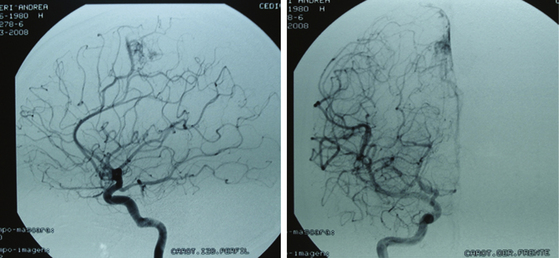
Radiosurgery is not indicated as a stand-alone option in this group of lesions. It can be considered a second-line treatment after endovascular therapy has been carried out, but it has been proved (as in AVMs of higher volume) that the area to be radiated does not include the whole nidus and that areas treated by endovascular means may not be included, thus leading to lesion reproduction in the long term.13,17,75 Even though the proposal to always treat grade I and II AVMs may be controversial, the specialized bibliography supports my strong recommendation for surgery (Fig. 83-2).2,5,6–19,21–23,25,29,31–37
Grade I and II AVMs have with surgery (alone or combined) a cure rate close to 100%.17,31,32,36,37,41,42
Grade III AVMs
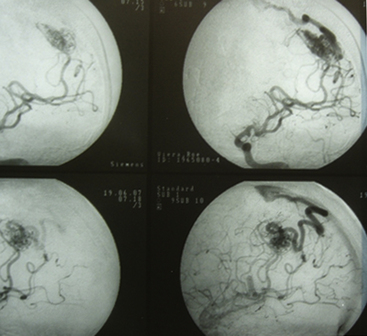
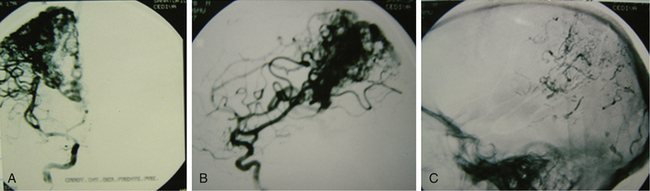
Grade III AVMs must always be treated. They can become highly problematic due to the extensive number of variants that can appear within this group. AVMs that have a large nidus, are superficial (corticosubcortical), and involve one or two vascular territories must always be treated by means of combined techniques. Endovascular therapy is essential in these cases (in either one or two sessions) and is aimed at progressively occluding the afferent vessels (through which inversion of the flow is achieved) but not causing a sudden redistribution of it, which could be harmful. Endovascular therapy with Onyx offers excellent results in this group of AVMs as a treatment prior to surgery.43,44 A prudent amount of time should elapse between this treatment and surgery (no less than 4 or 5 days). After the surgery, and by means of a control angiogram, the complete elimination of the nidus must be verified. If small remnants remain, the treatment can be complemented with radiosurgery.
Most lesions within this grade, when treated, have a cure rate close to 100% but a morbidity rate close to 25%.27,31,37 As with grades I and II, the preceding proposal may result in controversy, but the specialized bibliography recommends such treatment (Figs. 83-3 and 83-4).17,21–23,25,31–37,45
1. Endovascular therapy + surgery
3. Endovascular therapy + surgery + radiosurgery
4. Endovascular therapy + radiosurgery
Grade IV AVMs
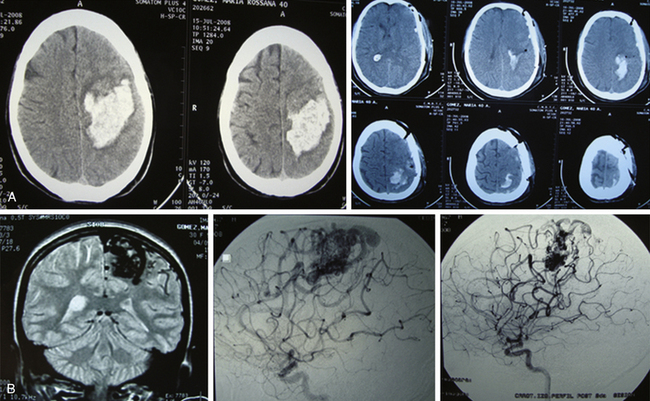
Grade IV AVMs that have not bled and do not have angiographic signs suggestive of complications (e.g., intranidal aneurysms) should be controlled with clinical and imagenologic evaluation.19,22,33,34 The morbidity and mortality rates of the treatment are higher than those supplied by spontaneous course. If there was hemorrhage, these AVMs must be treated, since it is well known that if a lesion of such characteristics has bled and the patient survived, the AVMs will bleed again. In addition, it is highly likely that after hemorrhage the patient will present with neurologic sequelae, which diminishes the rate of sequelae occurrence due to treatment.
When these cerebral vascular malformations are treated (using a combination of options), the cure rate is close to 100%, but the possibility of sequelae rises to almost 50%.17,21,22,32–34
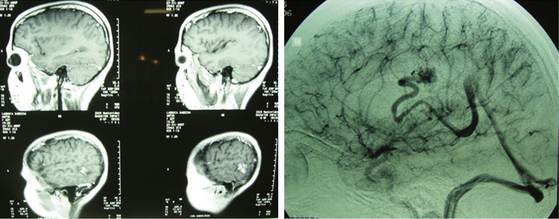
Sometimes a palliative treatment will be proposed: endovascular surgery in association with radiosurgery or as a stand-alone option. With such treatment, the possibility of bleeding is reduced but the patient is not necessarily cured (Fig. 83-5).
2. Endovascular therapy + surgery
3. Endovascular therapy + surgery + radiosurgery
4. Endovascular therapy + radiosurgery
Grade V AVMs
In principle, grade V AVMs are not treated.17,19,37 Risks are high; cure is possible, but at the cost of sequelae. If they are aggressive lesions, in the sense of presenting recurrent bleeding, treatments must be tried. In my opinion, those treatments will always be palliative and will be dominated by endovascular surgery and the possibility of being completed with radiosurgery. Direct surgery should not be an option in malformations of this grade. Regardless, a grade V malformation can be operated on, but always with the prior endovascular therapy as an adjuvant. Surgery will always be difficult. The sequelae rate is high. In my opinion, surgery of grade V AVMs must be reserved exclusively for those cases in which there is recurrent bleeding and the patient, already with sequelae, has a life risk in case of new hemorrhage.17,19,33,35,45–48 Some authors propose radiosurgery as the first step of treatment and then endovascular therapy. In all cases, it will be a palliative treatment (Fig. 83-6).17,21,22,37,46–48
2. Endovascular therapy + radiosurgery
4. Endovascular therapy + surgery
5. Endovascular therapy + surgery + radiosurgery
A recent publication49 studied the safety of surgery in the treatment of AVMs. The conclusion was that in grades I and II the surgical risk (morbidity) is under 1%, while in grade III to V AVMs it varies according to the involvement of eloquent areas; surgical morbidity and mortality range from 17% to 21%. The comments of Batjer and Hernesniemi on this study support surgery as the best option in the treatment of the AVMs.49
Preparation for Surgery
Unlike in other neurosurgical interventions, the preparation for AVM surgery requires the fulfillment of several indispensable steps that allow the surgical act to be performed with fewer risks. First, the surgical intervention of an AVM should always be scheduled. A patient with an AVM complicated by a hemorrhage may require an emergency operation—a case that supports such action. But the indicated action in these cases is to proceed exclusively to the hemorrhagic area evacuation and not seek the malformation in this urgent surgical act. A swollen brain, a distorted anatomy, vessels compressed by the hematoma, venous stasis, and associated ischemic phenomena due to several of these factors conspire in such conditions against the surgical attempt on the AVM, rendering it a surgical failure, not to mention affecting the vital and functional prognosis of the patient. The exception can lay in small malformations found in the wall of the hematoma. In such cases, the resection can be performed. Although a hemorrhagic lesion was evacuated and nothing was found on searching in its “walls,” the pathologist may later report that a small AVM was found among the clots (Fig. 83-7).
Second, when a malformation is going to be operated on, the patient must be prepared for surgery. This implies complete studies and, if necessary, the performance of endovascular techniques. When occlusions of afferent pedicles and part of the malformation nidus have been carried out by endovascular means, surgery must be performed no fewer than 4 or 5 days after the treatment was carried out.50,51 Apart from this requirement, the timing for the surgery depends on the characteristics of the AVM, its flow, its size, the afferents and efferents, and the grade of occlusion achieved. Most importantly, it depends on the patient’s clinical condition after the procedure. An asymptomatic patient can be operated on prematurely, but those who start to present a neurologic deficit and whose imaging studies reveal an ischemic lesion must be differed until clinical stabilization is achieved. Therefore, the surgical team, taking into consideration these factors, should determine the best moment for surgery in each case. The existence of an AVM provokes an alteration in the brain’s blood flow. The occlusion determines a new redistribution of the brain’s blood flow, regardless of the percentage occluded by endovascular means. Such modification, which has been progressive with the endovascular therapy, experiences an abrupt change with surgery. Thus, allowing time for the changes in blood flow to be induced by the therapy before surgery takes place reduces the possibility of associated morbidity.51