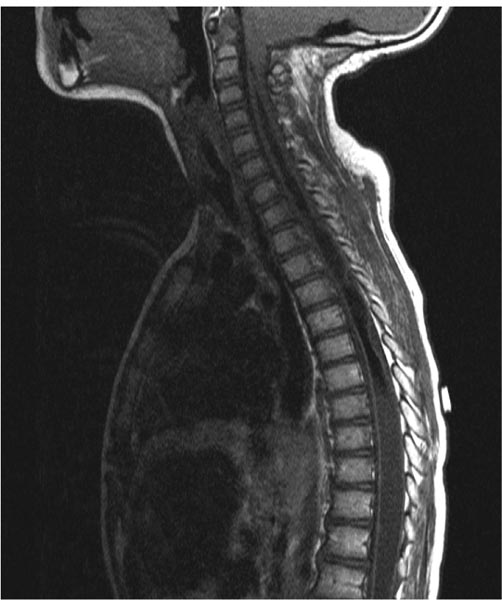
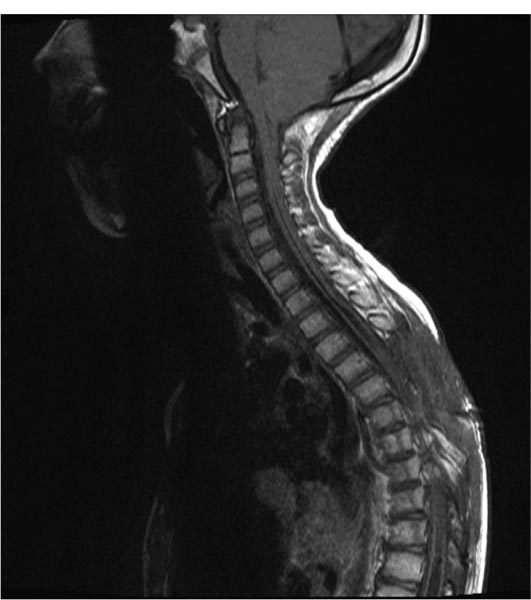
20 Syringomyelia Syringomyelia is a disorder of the spinal cord characterized by a fluid-filled cavity within the cord substance. When the cavity is a dilated central canal of the spinal cord, the term hydromyelia has been applied, reserving the term syringomyelia for cavities in the cord extending lateral to or independent of the central canal.1 The term communicating syringomyelia also refers to an enlargement of the central canal, whereas noncommunicating syringomyelia refers to a cyst not in communication with the central canal, but rather arising from the cord substance. The term syringohydromyelia reflects the difficulties in classification and terminology.2 Others have used the terms syringomyelia and syrinx in a general manner to represent any spinal cord cyst, and we shall also use these terms in the same general sense in this discussion.3 There is a prevalence of 8.4 cases per 100,000 population of syringomyelia related to nontrauma, according to Heiss and Oldfield.4 In patients with Chiari I malformation, the incidence and prevalence of syringomyelia vary depending on the series quoted. For example, between 3 and 75% will have a syrinx seen on magnetic resonance imaging (MRI).5–9 In a study by the senior author (RE), 80.5% of pediatric patients with Chiari I malformation had associated syringomyelia.10 Twenty to 95% of those with Chiari II malformations will have a syrinx on neuroimaging.11 In a study by Rossier et al, 3.2% of 951 patients with spinal cord injuries followed for 11 years developed syringomyelia.12 Heiss and Oldfield found that 4% of intramedullary spinal cord tumors have associated syringomyelia.4 Syringomyelia develops due to an alteration of cerebrospinal fluid (CSF) circulation at the foramen magnum or around the spinal cord.13 Important theories of syringomyelia formation include the water-hammer theory of Gardner,14–17 the craniospinal dissociation theory of Williams,18,19 Ball and Dayan’s theory involving Virchow-Robin spaces,20 and Oldfield et al’s theory of the cerebellar tonsils moving down with systolic pulses, producing a systolic pressure wave in the spinal CSF that acts on the spinal cord surface.21 Ellenbogen et al found that in patients having Chiari I malformation with and without syringomyelia, aberrant CSF flow profiles were observed at the craniocervical junction.10 A syringosubarachnoid shunt provides an outflow route for CSF during external cord compression, which occurs during cardiac systole.22 Communicating syringomyelia is usually observed with pathology involving the foramen magnum. Examples of this are Chiari I malformation and basilar arachnoiditis. Occult spinal dysraphism can also cause communicating syringomyelia. Noncommunicating syringomyelia can be caused by trauma, neoplasm, intradural arachnoid cysts, arteriovenous malformations, and arachnoiditis. The causes for syringomyelia are very diverse. Yet all of the conditions share a common feature: an alteration in the normal CSF dynamics in the spinal subarachnoid space.23 The classic description of the “syringomyelia syndrome” consists of a dissociative sensory loss (loss of pain and temperature sensation with relative sparing of light touch and perception) in a cape-like distribution with upper extremity weakness of a lower motoneuron type and lower extremity weakness of an upper motoneuron type.24 The clinical presentation can vary greatly and may include weakness, sensory loss, pain, gait abnormality, scoliosis, and changes in bladder or bowel function.25 Physical findings can include an ascending sensory level, increased motor deficits, depressed tendon reflexes, atrophy, increased spasticity, hyperhidrosis, autonomic dysreflexia, and myelopathy. First, one must determine if the syrinx is causing significant or progressive neurologic symptoms or deficits. If it is felt that the syrinx is responsible for the progressive symptoms or signs, one must always first attempt to discover and treat the underlying etiology of syringomyelia in the individual patient. There is no medical or pharmacological treatment for syringomyelia. Primary treatment of syringomyelia can include posterior fossa decompression in the setting of Chiari I malformation. Many surgeons argue that decompression of the posterior fossa to enlarge the volume of the posterior fossa at the craniovertebral junction and establish normal CSF flow patterns should be the primary goal of surgical intervention in patients with Chiari I malformation and syringomyelia. In patients who have undergone posterior fossa decompression and a symptomatic syrinx persists, reexploration can be considered after appropriate radiologic evaluation to look for an etiology such as a pseudomeningocele or obstruction to normal CSF flow. Shunting of the syrinx should be used only if the patient fails treatment of the underlying cause of the syringomyelia. A shunt cannot correct the initial pathophysiology of syringomyelia formation, and for this reason it is always best to aim treatment initially at the causative mechanism. It is also possible that a patient has a syrinx without an obvious cause. If no cause of a significant syrinx is found, consideration for exploration of the craniocervical junction may be considered in selected patients.26,27 Primary syringosubarachnoid shunting can also be useful for this population. Shunting procedures of the syrinx as the initial mode of treatment for Chiari I malformation have a relative contraindication because of concern that further herniation by spinal cord collapse and compromise of the brainstem will be precipitated by such a procedure.28–30 Van den Bergh found that in patients with Chiari I malformation, if only a syringosubarachnoid shunt is inserted, the headache attacks may become more severe, because the shunt makes the craniospinal pressure difference even greater.31 There also can be a danger of cerebellar coning with apnea. These observations do not uniformly hold for children with Chiari II malformations. A syringosubarachnoid shunt should not be chosen as a first-line treatment for syringomyelia. Its use should be considered only after failed attempts to treat the underlying pathophysiology of the syrinx. Use of the syringosubarachnoid shunt also is dependent on a normal flow of CSF in the subarachnoid space. Hence, patients with a history of arachnoiditis may not be candidates. A person with a history of chemical or tuberculous meningitis can have diffuse arachnoiditis and is thus not an ideal patient for syringosubarachnoid shunting. Arachnoid flow can be significantly altered in post-trauma patients; thus, this population may be less responsive to syringosubarachnoid shunting. In patients with prior arachnoiditis and trauma, it is possible that the distal shunt should be positioned in the peritoneum or pleural space, rather than in the subarachnoid space. Recognition of septa within the syrinx on MRI may hinder drainage through a shunt.28 This therefore can also be considered a contraindication to shunting procedures. However, free communication within the syrinx can exist despite septation seen on MRI.32 Intraoperative neurophysiologic monitoring is set up after the patient is anesthetized. This includes motor evoked potentials and somatosensory evoked potentials (SSEPs). The patient is positioned prone on bolsters on the operating room table. Towel, foam, or gel rolls are used to serve as bolsters in the adolescent patient. The arms are positioned up at 90-degree angles if operating on the thoracic or lumbar region. The arms are placed at the patient’s side if the syrinx is located at the cervical region. All pressure points are adequately padded. A single prophylactic dose of antibiotics is administered by the anesthesiologist 30 to 45 minutes before skin incision. A laminotomy is planned at the levels with the largest cord expansion caused by the syrinx. Intraoperative fluoroscopy is first used to mark the relevant levels of the spinous processes, to plan the extent of the initial skin incision. Subperiosteal dissection is then continued to complete the exposure of spinous processes and lamina. A Leksell rongeur, Kerrison punch, and/or high-speed drill is used to perform the laminotomy. Conservative bone removal is employed to avoid iatrogenically induced instability. Intraoperative ultrasound may be used to confirm the underlying syrinx and define its borders. The microscope is brought into the field at this point to open the dura. Special care is taken not to violate the arachnoid layer. The dura is tacked laterally using 4–0 Nurolon sutures. A beaver or arachnoid microblade is used to vertically cut the arachnoid and pia in the midline or at the dorsal root entry zone (DREZ). Often, the surgeon chooses the region with the thinnest and most transparent trajectory to the syrinx-filled cord. Care should be taken to maintain the subarachnoid space. Hemostasis is important so blood does not track in the subarachnoid space. The blade is then carefully used to perform a 2 mm midline or DREZ myelotomy and then enter the syrinx. Once the syrinx is entered, fluid rushes out, decompressing the syrinx. We use a K-shaped syringosubarachnoid shunt made of nonreactive Silastic of the Pudenz type (Medtronic PS Medical, Goleta, California). This has multiple fenestrations at the ends. One end of the K is tunneled into the syrinx rostrally. Another end is placed caudally in the syrinx. The distal end of the K tube is placed in the subarachnoid space and anchored to the pia at the myelotomy site with a 6–0 Prolene suture. It is important that the distal end of the K tube be placed in the subarachnoid space in order for effective absorption to occur. The dura is closed using fine running suture. A standard multilayered closure of the operative site is then performed. The patient is kept on flat bed rest for 48 hours to minimize the chances of a postoperative CSF leak. Postoperative MRI is conducted within 48 hours to check for a collapsed syrinx. Figure 20.1 is a preoperative sagittal T1-weighted MRI of one of our patients showing a large syrinx. This 8-year-old boy already had two posterior fossa decompressions. He presented again with progressive weakness in his hands and more difficulty with ambulation. We placed a syringosubarachnoid shunt, which has arrested progression of his neurologic deficits as of 1-year follow-up. Figure 20.2 shows the collapsed syrinx on the second postoperative day. Fig. 20.1 Preoperative sagittal T1-weighted magnetic resonance imaging (MRI). Fig. 20.2 Postoperative sagittal T1-weighted MRI. Rhoton advocated that the myelotomy and placement of a cavity to subarachnoid shunt should be made in the DREZ because this is the thinnest portion of the spinal cord when cavitation takes place.33 Theoretically, this prevents loss of bladder control and prevents neurologic deficit.6 We agree with Menezes, who noted a paucity of neurologic deficits with midline myelotomy when this has been the thinnest portion of the spinal cord in a distended hydromyelic sac.6 We present, in chronological order, the highlights of several studies that discuss the use of syringosubarachnoid shunts in pediatric patients. 1. Vaquero et al described a 14-year-old male patient with syringomyelia who had a 1-year history of dissociated sensory loss and light spasticity in the legs. A syringosubarachnoid shunt was placed, and at the 2-year follow-up he had subjectively improved.34 2. Dauser et al reported on five pediatric patients who underwent syringosubarachnoid shunt placement.35 One patient had a syringosubarachnoid shunt placed at age 3 that improved his symptoms (a myelogram had showed low cerebellar tonsils and a wide cord from C2 to T2). Five years later, he had rapid acceleration in scoliosis, and 3 years subsequently, he developed upper extremity weakness. The child, then 11 years old, had posterior fossa decompression. This stopped the neurologic progression, and the child remained stable 2 years later. In the four other pediatric patients, ages 4 to 11 years, posterior fossa decompression and syringosubarachnoid shunting were performed. The authors reported good outcomes in these four cases. Pain resolved, and there was no scoliosis progression. Follow-up on these patients ranged from 16 months to 2.5 years. 3. Phillips et al reviewed four children ages 3, 5, 15, and 15 with scoliosis and syringomyelia, three of whom were followed to skeletal maturity.36 Each patient had the syrinx drained by a syringosubarachnoid shunt. The average follow-up was 8 years. Three of the four patients’ neurology improved after drainage. The other patient was stabilized. The older two patients had spinal fusions after the drainage operations. Fusions were felt to be less risky after syringosubarachnoid shunt placement. One child had kyphosis at the laminectomy site in follow-up. Progression of scoliosis was arrested only temporarily by syrinx drainage in the 3- and 5-year-olds. The authors concluded that long-term follow-up shows progression of scoliosis even with drainage of the syrinx plus management with orthotics. 4. Nagib described one pediatric patient with a Chiari I malformation and syrinx that were treated by a sub-occipital craniectomy but later required syringosubarachnoid shunting, due to the generous nature of the syringomyelic cavity.37 Resolution of the cavity was noted. Details of the case were not described. 5. Sgouros and Williams found an approximately 50% rate of longevity for 73 patients who had a syrinx shunt procedure.38
Syringosubarachnoid Shunts
Epidemiology
Pathophysiology
Clinical Presentation
Indications
Contraindications
Operative Procedure
Results
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree