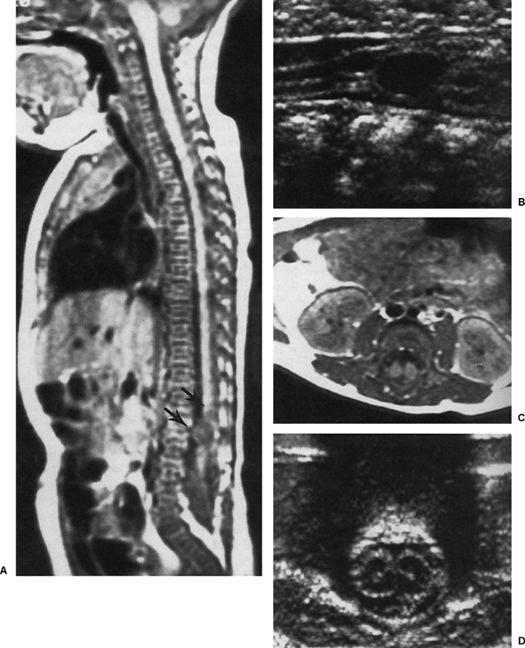
30 The Perinatal Management of a Child Born with a Myelomeningocele J. Grant Buttram Jr., Frederick A. Boop, and Charles Teo It is germane that the treatment of the child born with a neural tube defect is included in a textbook of neurosurgical emergencies. Although most neurosurgeons would agree that the operative repair of a leaking myelomeningocele is better classified as “urgent” rather than “emergent,” central nervous system (CNS) infection remains one of the leading causes of death in the perinatal period in infants born with neural tube defects. Evidence also suggests that those who survive the infection may have long-term cognitive dysfunction secondary to the ventriculitis.1 Measures begun immediately following delivery can effectively reduce the risk of a perinatal infection. Furthermore, evidence suggests that prenatal diagnosis and the mode of delivery may impact upon the long-term prognosis of the spina bifida child. Spina bifida is the most common congenital abnormality of the CNS. Its incidence varies between 1 and 5 per 1000 live births, depending upon race and demographics.2,3 Epidemiological and laboratory studies have cited a variety of causes for spina bifida. Certainly, genetics appears to play a significant role because the incidence of a neural tube defect increases 20-fold if a sibling has been affected.4 Other hypotheses include maternal pyrexia, ingestion of blighted potatoes, retinoic acid, viruses, anticonvulsants, and deficiency in maternal folate.5 The fact that the incidence of spina bifida increases in lower socioeconomic groups and in mothers with poor nutrition argues in favor of a nutritional component to the development of the disease.6 In fact, double-blind randomized and nonrandomized studies have shown a 70% reduction in the recurrence rates for mothers of spina bifida children given folate supplements as compared with similar mothers given a placebo.7 Subsequent studies have demonstrated a 60% reduction in first-time dysraphic births in mothers receiving similar supplements.8 The vitamin supplements were instituted 28 days before intended conception and continued until the second missed menstrual cycle. Certain anticonvulsants have been associated with higher incidence of myelomeningocele, possibly through their effect on folate metabolism. Studies have shown a lower incidence when both the number and the dosages of the drug are decreased before intended conception.9 The United States Public Health Service recommends that all women of child-bearing age who are capable of becoming pregnant consume 0.4 to 1.0 mg of folic acid per day.7 Given that more than half of the pregnancies in our country are unplanned, it would only be through general dietary supplementation that a reduction in the incidence of spina bifida through this mechanism could be accomplished. Perhaps no other disease in neurosurgery has received as much attention concerning whom and when to treat as has that of the child born with the Arnold-Chiari type II malformation, or spina bifida aperta. This has led some pediatric neurosurgeons to look for elements within the disorder that might influence outcome as a means of determining which children should be treated aggressively and which children should be allowed to die.10,11 The most notable such study was that of Lorber, published in 1971.12 He categorized patients into two groups, with paralysis at or above L2, marked hydrocephalus, kyphosis, and other congenital anomalies or birth injuries being used as criteria for nonsurgical management. His results demonstrated that of those with adverse criteria, half died. Forty percent had a normal intelligence quotient (IQ). Of those who had no adverse criteria, one fourth died, half had severe sequelae, and 14% were mentally deficient. He surmised that treatment should be offered only to those patients who could “look forward to life without grave handicaps.” This study has been refuted by McLone, who has demonstrated that these selection criteria are unable to predict which patients will have a normal intelligence or be productive, and therefore should not be used. In his study, in which 89 children had aggressive care, including repair of the myelomeningocele within the first 24 hours of life, the surgical mortality was 2% and the overall mortality at a minimum of 31/2 years of follow-up was 14%. In this population, 80% required shunting, 73% had a normal or above-average IQ, 54% were able to ambulate, and 87% achieved social urinary continence.13 Lorber’s proposed selection criteria have subsequently fallen by the wayside. Currently in North America, most pediatric neurosurgeons would agree that there are no good selection criteria upon which to base a decision for nontreatment of the child with spina bifida. This chapter offers specific guidelines as to the perinatal management of these children based upon our current state of knowledge and current societal trends. The wider utilization of prenatal ultrasonography and screening of high-risk pregnancies has resulted in the earlier diagnosis of spinal dysraphism.14 Once the diagnosis has been confirmed by maternal serum α-fetoprotein analysis, decisions regarding the future management of the pregnancy should be preceded by consultation and discussion with pediatricians, neurosurgeons, and geneticists. Options include termination of the pregnancy, early intervention, or no intervention. Progressive hydrocephalus is uncommon in the fetus and appears to be more likely to occur the higher the spinal level of the defect; however, if there is increasing ventriculomegaly, early intervention may take the form of cesarean section of the preterm infant once the lungs have reached maturity. Should fetal distress occur prior to lung maturation, cesarean delivery may still be warranted following appropriate consideration of the complex ethical issues. The once actively debated issue of in utero diversion of cerebrospinal fluid (CSF) via ventriculoamniotic shunt has since been shown to produce minimal, if any, benefit and has mostly been abandoned.15 Once pregnancy has been allowed to continue to term, then the mode of delivery becomes an issue. It has been demonstrated that the mode of delivery does not affect intellectual outcome in these infants.16 Another prospective nonrandomized study conducted by Luthy et al confirmed this, but also demonstrated that children with a neural tube defect who were exposed to the forces of labor were 2.2 times more likely to have a severe paralysis than were those delivered by cesarean section before the onset of labor.17 This suggests that the forces of labor and delivery may be harmful to the exposed spinal neural elements, and has been the rationale behind recommending elective cesarean section for mothers of infants in whom the lesion is recognized antenatally. Studies have not been conducted examining the possible relationship between mode of delivery and perinatal CNS infections in children with a myelomeningocele born to mothers infected with group B streptococcus. However, it is not difficult to imagine that such a relationship might exist, lending further credence to elective cesarean section in the possibility of delivering a neonate with an open neural tube defect. If the diagnosis of myelomeningocele has not been made prenatally, it generally becomes obvious upon inspection of the newborn. Three important questions then need to be addressed. First, does the fluid space communicate with the environment? Although in the authors’ experience the majority of these lesions leak CSF, it can sometimes be difficult to establish this on initial examination. Careful inspection of the child while crying, or gentle pressure on the anterior fontanelle may reveal CSF leaking from the lesion. Palpation of the lesion itself or probing with an instrument is neither helpful nor recommended. A sterile gauze placed over the lesion during the remainder of the examination may become moist with serum of CSF, but frank wetness establishes the diagnosis of an “open” lesion. Closed lesions are generally accepted to be treatable electively, the feeling being that the risk of ventriculitis associated with CSF leakage does not exist. The second question to be answered concerns the neurological status of the child. Determining the exact level of neurological dysfunction can be difficult. One should be aware that signs may be complicated by a combination of myelopathic and radiculopathic damage, and possibly by an element of spinal shock. Stimulation, either by sound or by touch, may elicit reflex movements that give the parents a false sense of optimism. In fact, simple observation is often the best estimate of the child’s functional level. The rostral end of the lesion usually correlates with the level of neurological function. The orthopedic deformities, which result from the unopposed actions of certain muscle groups, may be of localizing value. Lesions above T12 will cause flaccid hips, legs, and feet. Lesions below L1-L2 will cause fixed flexion deformity of the hips due to functional iliopsoas unopposed by the gluteal musculature. Lesions below L3-L4 will result in genu recurvatum, and lesions below L4-L5 may cause degrees of talipes equinovarous or pes cavus. All patients with spinal dysraphism are presumed to have some degree of neurogenic bladder dysfunction. In the presence of a dyssynergic bladder, the practice of trying to expel urine by suprapubic pressure (Credé’s maneuver) may cause ureteral reflux and is to be discouraged.18 The third question to be addressed regards the presence of associated congenital anomalies. Ten percent of children with spina bifida will have a chromosomal abnormality, and 15% will harbor other anomalies outside of the nervous system.17,19 A thorough examination of the cardiovascular, gastrointestinal, and pulmonary systems is mandatory before consideration can be given to surgical intervention. Within the nervous system itself, greater than 80% of these children can be expected to have hydrocephalus, and 90% will have an associated Chiari II hindbrain abnormality. Tandem abnormalities of the spinal cord such as diastematomyelia, syringomyelia, dermoid tumors, or spinal arachnoid cysts are not uncommon. When the head is large, the scalp veins dilated, and the fontanelle full, the diagnosis of hydrocephalus is easily made; however, hydrocephalus may exist in the absence of clinical signs, being recognized only by the sonographic presence of ventricular enlargement. In some instances, it may not develop at all until several days following repair of the myelomeningocele. Similarly, the clinical manifestations of the Chiari II hindbrain malformation may be subtle and may not become apparent for months to years following birth. Whether stridor, poor feeding, lower cranial nerve palsies, or apneic spells are due to direct compression of the brainstem by the herniated cerebellar tonsils, or are secondary to intrinsic malformation of the medulla may be difficult to discern. Hence, which symptoms will respond to decompressive surgery remains a matter for contention. In general, brainstem dysfunction is a poor prognostic sign. Of the 14% to 18% of these children who die beyond the perinatal period, most will die from complications of the hindbrain malformation.20,21 Not uncommonly, a neurosurgeon’s first exposure to a child with a neural tube defect will have resulted from a fetal ultrasound. The favored imaging modality for fetal imaging due to its sensitivity in detecting tissue-water interfaces, and its low radiation exposure to the fetus, the fetal ultrasound serves as an excellent tool in the early detection of neural tube defects.15 Signs of myelomeningocele seen on ultrasound prompt a more detailed examination of the fetus. Biochemical markers, fetal chromosomal studies, and MRI are all appropriate tools in the antenatal diagnosis of myelomeningocele and may be useful in the diagnosis of other developmental abnormalities. When considering preoperative investigations of the newborn child with spina bifida, one should keep in mind the principles of minimal handling and patient comfort. Laborious, time-consuming, costly, and invasive tests are unnecessary in the immediate management of the infant with spina bifida aperta. However, a plain chest x-ray is simple to perform and offers important information that may be essential to the surgeon and the anesthesiologist. The same x-ray is often taken to verify the placement of an umbilical vein or artery catheter and can incorporate the spinal column at the level of the lesion to demonstrate the presence and severity of an associated spinal deformity. An ultrasound of the head serves as an excellent baseline examination of the ventricles and may provide accurate images of the posterior fossa. Transcranial Doppler flow studies of the major branches of the circle of Willis may also reflect abnormal cerebral perfusion in the face of progressive hydrocephalus because resistance to blood flow in these vessels increases in the face of increasing intracranial pressure.22 An ultrasound of the spine in these neonates may demonstrate the presence of syringomyelia, diastematomyelia, or a dermoid tumor, and can accurately determine the level of the conus medullaris (Fig. 30-1).23,24 At the same time, the kidneys and bladder can be visualized, revealing the number and position of the kidneys and the presence of hydronephrosis, or an overdistended bladder. When considering antenatal counseling it is clear that the neurosurgeon is of key importance and is an integral member of the counseling team. The neurosurgeon offers an intimate relationship with the condition including a detailed knowledge of the structural abnormality, experience regarding related conditions, and information on long-term quality of life issues through the treatment of patients at various stages of life. Other members of the counseling team should include a genetic counselor, a perinatologist or pediatrician, and an obstetrician. A team composed of these members will be able to provide detailed information in an impartial manner to the family regarding the nature of the condition, obstetric management, postnatal care, quality of life issues, and any legal issues regarding elective termination because a large number of pregnancies affected by spina bifida are terminated electively. In fact, it is estimated that as many as 50% of pregnancies affected by neural tube defects and nearly one quarter of those affected by spina bifida are terminated electively.25,26 In counseling the family, all of those involved need to be constantly reminded that the stress on the family and the burden on the health care system merely begins once the spine lesion is repaired. Treatment of this condition comes at a substantial cost both to the family and to the health care system. In fact, costs have been estimated to exceed $340,000 per lifetime per patient and an annual cost of nearly $500 million to the health care system.27,28 These children require lifelong multidisciplinary support, sometimes in an institutionalized environment. By spending time with the family in these early states, educating them, and showing empathy, one may cultivate understanding and acceptance of this chronic affliction. This, in turn, will create less strain on the family, better acceptance of the child, fewer institutionalized children, and, ultimately, less burden on the health care system. It has been demonstrated that parental satisfaction with, acceptance of, and responsibility for the child are directly related to the quality of information given and their degree of involvement in the decision-making process.29 When counseling parents, it is important to minimize feelings of urgency, and to explain truthfully and clearly the ramifications of having a child with this condition. Understand, however, that parents faced with the birth of a less-than-perfect baby are unlikely to properly assimilate information presented to them. Indeed, a survey by McLone points out that they do not fully understand the nature of their child’s affliction until as long as 6 months after the child’s birth.30 In this regard, they ultimately must rely upon the advice of their physician. The prognosis of children with spina bifida varies dramatically with the level of the lesion and the presence of associated anomalies, but several generalizations can be safely presented. Current data suggest that more than 90% of newborn infants with spina bifida aperta will survive beyond infancy. Three out of four will have normal intelligence, more than half will walk with or without some form of assistance, and nearly 90% will achieve urinary continence with the use of drugs and intermittent catheterizations.20
Prenatal Diagnosis
Postnatal Diagnosis
Neuroimaging
Counseling and Timing of Surgery
The Perinatal Management of a Child Born with a Myelomeningocele
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








