Fig. 1.1
(a) Gadolinium-enhanced, T1-weighted axial MRI of a vestibular schwannoma on the left side. The primary data for image guidance in this particular case were obtained from both a spiral computed tomography and magnetic resonance imaging. (b) Semi-sitting position of the patient for the retrosigmoid approach. The infrared camera of the image guidance system is placed low on the side of the lesion so that it can “see” the probe. (c) The course of the transverse and sigmoid sinuses and the bone sutures meeting at the asterion has been drawn to the skin with a surgical marker. (d) Corresponding image on the navigation screen with the virtual probe pointing to the transverse-sigmoid transition
Several volumes of interest may be selected and colour-coded to create a volumetric image that contains all the anatomical landmarks that would also appear in the real operating field. This image – the “virtual operating field” (VOF) – on advanced image guidance systems can be interactively rotated to match the orientation of the real surgical field, as seen through the microscope (Fig. 1.5). Surfaces may gradually be rendered translucent in the image to allow a view of the lesion in relation to more superficial morphological landmarks (Figs. 1.1, 1.2, and 1.3, “driver seat view”). The VOF ideally should be zoomed to the size of the real microscopic field, and both views may be displayed on a video monitor or in the ocular of the surgical microscope simultaneously. Depending mostly on the quality of the imaging data and the level of the human computer interface (intuitive software, computer skills of the surgeon), image rendering may take between 15 min and 2 h.
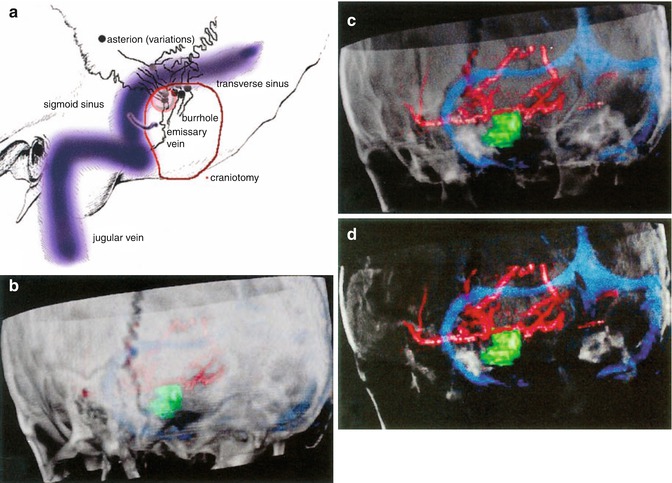
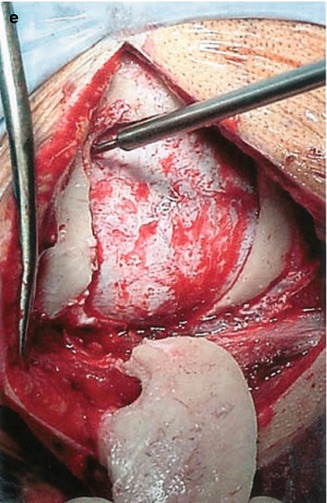
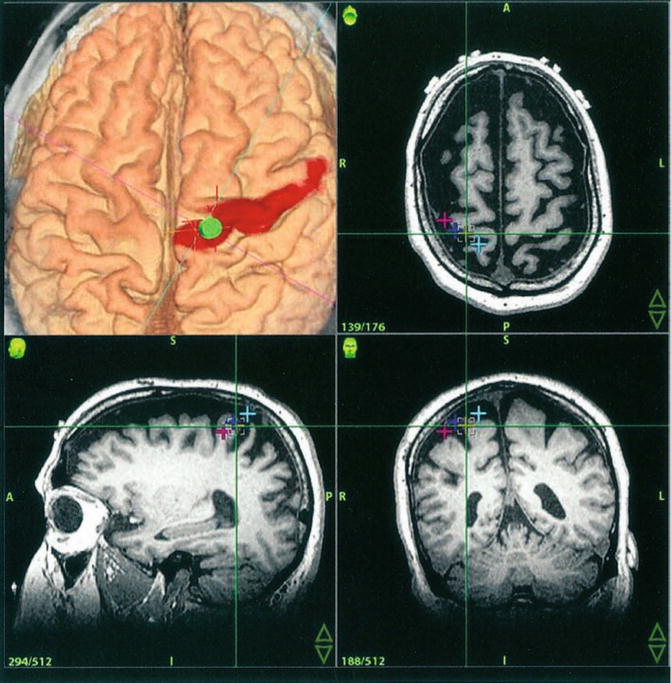


Fig. 1.2
(a) Anatomical landmarks for the lateral suboccipital approach. Note the variability of the location of the asterion around the sinus transition (b) 3D rendering of the bone sutures of the posterior fossa with the navigation system. (c, d) Gradual transparency modulation of the bone allows for a look through the posterior fossa. The borders of the venous sinuses become clearly visible so that they can be used as landmarks themselves, replacing the asterion as a historical landmark. The circle of Willis is also shown in the images. (e) After a burrhole has been placed just below the transverse-sigmoid junction, an osteoplastic lateral suboccipital craniotomy is carried out

Fig. 1.3
Image guidance with 3D renderings (upper left) and tri-axial MR-images in a patient with intractable thalamic pain during implantation of a motorcortex stimulator. The visualization of the cortical surface with its gyri and sulci is clearly superior in the volumetric 3D rendering. The motor strip has been segmented manually based on fMRI images of the patient. The virtual probe points – just as the real one – to a previously defined electrode location
In the operating room, the patient’s head is registered either with 5–10 adhesive skin fiducials and a pointing probe (which may be substituted by the microscope in some settings) or with a device and algorithm for surface detection. Most systems today are based on infrared transmission employing a camera for detection of a digital reference frame and the instruments (Fig. 1.4).
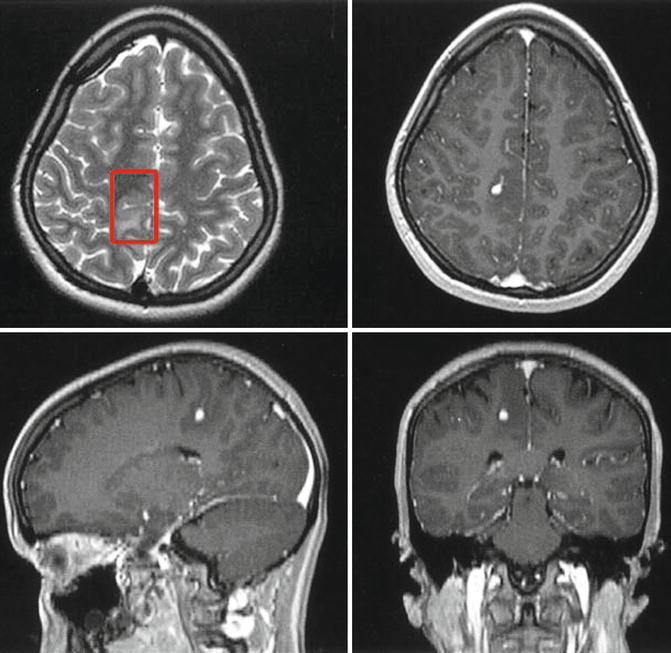

Fig. 1.4
An infantile desmoganglioma in the right postcentral region of a 14-year-old patient. The T2-weighted MRI (upper left comer) shows that the lesion centres around a sulcus. In the depth, nodular contrast enhancement in T1-weighted images points to a histologically dubious portion of the tumour
The digital reference frame (DRF) may be attached to the head holder, to the operating table, or directly to the head of the patient. It is kept visible by the camera by draping it with a transparent bag or by mounting it sterile on an appropriate extension.
After registration, a quick check should be performed to assess the accuracy of the registration by targeting a well-defined landmark (e.g. the nasion or the outer ear canal).
The quality of VOF images related to their closeness to reality will obviously depend on the quality of the primary data set. A contrast-enhanced target located adjacent to the bone and targets located in the middle fossa or at the craniocervical junction may pose difficulties because of decreased tissue contrast and MR image distortion. Also, due to the smaller sulci and the lesser amount of cerebrospinal fluid contained in the subdural space, the surface of the temporal lobe cortex is usually harder to be depicted in three-dimensional renderings.
The surgical approach is facilitated by VOF images by showing hidden landmarks like the transverse and sigmoid sinus in a retrosigmoid route [5, 7, 8] (Fig. 1.2).
Gradual modulation of the opacity of surfaces in VOF images allows for visualization of hidden anatomical structures and also for relating those structures to more superficial landmarks that were already within the surgeon’s view (like the asterion in Figs. 1.2 and 1.4).
With 3D renderings there is no need for the surgeon to mentally reconstruct the surgical anatomy from two-dimensional scans. Compared to tri-axial 2D images, orientation is significantly faster and more comprehensive with VOF images (Figs. 1.1, 1.2, and 1.3). This becomes more apparent when irregular-shaped structures like the dural sinuses, the basal arterial circulation, the bone of the skull base, tumour borders, and the cortical surface are involved or when the operating field was rotated into an unusual orientation [6, 39].
Advantages of image guidance multiply when functional and/or histological characteristics are added to a VOF. Figure 1.3 shows how functional image guidance aids in the placement of epidural electrodes for motor cortex stimulation in intractable pain. The morphological outline of the precentral gyrus can easily and safely be traced in 3D images if functional data are available. Precise electrode implantation with this guidance is greatly facilitated even with the dura remaining closed throughout the procedure [5].
Similar advantages have been reported for subdural placement of electrode grids in patients with epilepsy for the detection of the origin of focal seizure activity [29, 41].
Some brain tumours even though they reach the surface of the cerebral cortex do not show any demarcation to normal brain parenchyma (Figs. 1.1 and 1.5).
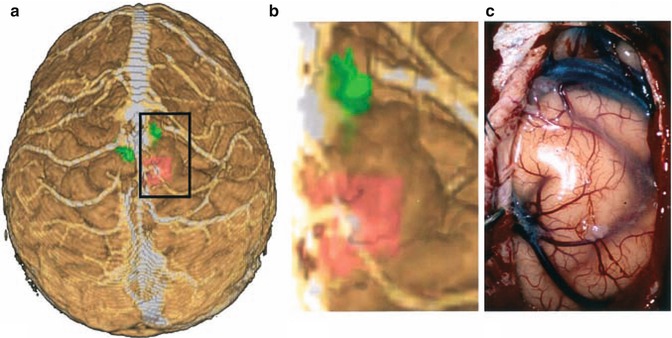

Fig. 1.5
(a) “Helicopter view” of the lesion and the motor areas for the left (pushed anteriorly) and right foot in a 3D rendering created from MPRAGE sequences and fMRI on the image guidance system. (b) The virtual operating field (“driver seat view”) showing the cortical surface and veins, the lesion, and the adjacent motor areas (shaded). Note that the latter two are exclusively seen in the VOF, but not in the real field. (c) Only the veins and the cortical surface can be recognized after the parasagittal craniotomy. Image injection of the VOF with a tumour outline and functionally significant areas can greatly enhance the real operating field in cases like this
However, if a tumour is well delineated on T2-weighted MR images (Fig. 1.5), its outline can be mapped or overlaid onto the cortical surface in a VOF. This is especially useful if the tumour is located adjacent to a functionally eloquent area, and therefore, resection has to be restricted precisely to the tumour limits in order to avoid intolerable morbidity. Functional imaging provides further information that would not be available in the real surgical field without neuronavigation (Fig. 1.1).
The functional anatomy can be shifted or distorted by the tumour. This is especially important when fibre tracking by diffusion tensor imaging is employed. The pyramidal tract may be considerably displaced and then returned to its normal position as tumour resection progresses. Under these circumstances of major brain shift, MR images acquired prior to the procedure would not be reliable during surgery. It has been shown that intra-operative functional MR imaging is feasible with the same protocols that are used outside the operating theatre. The combination of neuronavigation and intra-operative fMRI offers additional safety in these cases [11, 29–31]. Multimodal imaging may also improve reliability of functional information especially with respect to language areas of the cortex [8, 10, 32].
Zooming in the virtual images in order to enlarge the detail to the size of the surgical field reduces the amount of information presented at a time to the required minimum, steadying the surgeon’s focus of attention (Fig. 1.1).
Individual, patient-specific anatomy can be visualized in the VOF down to a resolution of about 2 mm for well-delineated structures. In general, all morphological structures that are readily discernable in primary imaging data can be also detected in virtual 3D models of the surgical situs. Using image fusion techniques, bony surfaces, embedded vessels, and different types of soft tissue along the surgical path, both in front of and beyond the lesion, can be identified in a single image, which in turn can be rotated to match the view through the microscope onto the real surgical field.
Stereoscopic images are not obtained as easily, but they have the decisive advantage of conveying information on depth along the z-axis [12, 13, 19, 35, 41] which can only be captured in tri-axial images with volumetric pseudo-3D rendering.
Smaller and less discernable structures, such as most cranial nerves are not well delineated in routine images. Electrophysiological monitoring still is by far more effective for early identification of the cranial nerves.
1.4 Outlook
Microneurosurgery depends on three-dimensional, stereoscopic information on the surgical field delivered through the microscope. A “virtual microscope” operating in 3D space that uses stereoscopic radiographic images of the patient’s anatomy would be an ideal instrument to plan these procedures and to obtain on-line information beyond the operating field during surgery. While several problems remain to be solved [18, 27, 34], true 3D imaging, on a routine basis, will probably be performed by taking advantage of stereoscopy in the future.
Overlay of a VOF that virtually matches the surgical field will certainly augment the surgeon’s capacities.
While anatomical knowledge and experience still remain the most crucial factors affecting the surgical result, virtual reality can provide additional information about elements in the operative field that are beyond the superficial layer and invisible through the operating microscope.
Although now in extensive clinical use, image guidance still is often perceived as an intrusion into the operating room [34].
In our experience, image guidance is best accepted when additional preparation time is minimal and the VOF is adjusted to the size and orientation of the real surgical field containing relevant landmarks without redundant information.
The following list is a brief compilation of essential prerequisites that – according to the literature and to our own experience – will have to be met in order to turn image guidance into an integral part of most microneurosurgical procedures.
1.
Accuracy: Image guidance needs to be precise. Geometric distortions in imaging procedures have to be corrected before the data are taken to the operating room.
2.
Easy and speedy applicability: Imaging, data transfer, segmentation, intra-operative setup, and registration combined should require minimal additional time.
3.
Image fusion: Multimodal images must be easily co-registered and combined in a single image.
4.
Truly stereoscopic 3D images: Stereoscopic visualization improves perception and enhances the ability to understand complex 3D anatomy.
5.
Interactivity: The practical benefit of 3D display is increased considerably when the size and orientation of the VOF correspond to the real microscopic view of the surgical field. Different perspectives of the field (driver’s seat view, helicopter view) should be optional. With respect to the limitation of the VOF, less may often be more, since the surgeon will only appreciate relevant information on the surgical field in view under the microscope.
6.
Transparency: The possibility to see through surfaces by gradually rendering them translucent is advantageous since landmarks at different depths along the surgical path can be correlated to one another.
7.




Integration of functional characteristics of tissue: Data from functional MRI, including fibre tracking by diffusion tensor imaging as well as PET, EEG, MEG, spectroscopy, etc., should all be made available in images used for intra-operative guidance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








