INTRODUCTION
Dystonia is defined as a syndrome with sustained muscle contractions that often result in twisting and repetitive movements and abnormal postures. Lacking a curative therapy, the treatment of dystonia is directed toward improvement in symptoms. Treatment goals include improvement of abnormal postures, reduction in pain and enhancement in functional capacity and quality of life. Over the past 10 to 15 years, the treatment of dystonia has undergone marked changes with the introduction of botulinum toxins (BoNT) and deep brain stimulation (DBS). Both of these treatment modalities have been investigated in various types of dystonia using placebo-controlled study designs that demonstrate efficacy. There has not been substantial progress for the pharmacologic and nonpharmacologic treatments for dystonia. This can be attributed to the lack of an understanding of the basic pharmacology of dystonia, the failure to identify specific neurotransmitter abnormalities that could provide targets for pharmacologic interventions, the heterogeneity of dystonia, the relatively low prevalence of dystonia, and the availability of other effective interventions (BoNT and DBS).
NONPHARMACOLOGIC INTERVENTIONS
The nonpharmacologic interventions for dystonia are based on clinical observations and current understandings of the neurologic mechanisms of dystonia. The pathophysiologic basis for dystonia includes a loss of inhibition, abnormalities in sensory function, and alterations in synaptic plasticity (1). These combined abnormalities have led to several innovative approaches to the treatment of dystonia. However, most studies evaluating this approach are open-label, providing class IV evidence.
Sensory tricks improve dystonia in approximately 70% of dystonia patients, although the percentage varies considerably depending on the study (2). Optimizing the effect of sensory “tricks” (geste antagoniste) has been proposed as a potential therapy for dystonia (3). Examples include appropriately fitted ankle–foot orthotic for lower-limb dystonia (4), dental devices for oromandibular dystonia (5), a variety of writing devices for writer’s cramp (6–8), and tinted lenses for blepharospasm (9). Occupational therapists with knowledge of dystonia may be able to optimize sensory tricks and reduce the need for more invasive therapies. Physical therapy as a treatment for dystonia has been described in open-label studies (10), but controlled studies are needed (11).
REPETITIVE TRANSCRANIAL MAGNETIC STIMULATION
Repetitive transcranial magnetic stimulation (rTMS) provides a method for producing cortical effects that are either excitatory (at high rates of stimulation: >5 Hz) or inhibitory (at low stimulation frequencies: <1 Hz) (12). These excitatory or inhibitory effects can be directed toward particular cortical regions through appropriate placement of the TMS coil. Application of inhibitory stimulation using rTMS at 1 Hz to the hand area of the contralateral motor cortex (MC) in 16 patients with simple or dystonic writer’s cramp showed improvement in handwriting in 8 of 16 patients, with marked improvement in 6. The benefit in these patients was sustained for more than 3 hours, with two patients having persistent improvement after several days. Sham stimulation with the coil placed anterior to the MC did not result in any improvement (13). A subsequent study in nine right-handed patients with dystonic writer’s cramp using rTMS at 0.2 Hz delivered in random order to the MC, premotor cortex (PMC), and supplementary motor cortex (SMC) showed significant improvement following PMC stimulation but not after SMC, MC, or sham stimulation. A recent study demonstrated using a blinded, controlled design that pianists with dystonia did not improve with bihemispheral transcranial stimulation, but if TCA was combined with behavioral therapy, there was significant improvement that persisted for 4 days after stimulation (14). This technique may be promising for blepharospasm and writer’s cramp (15–17).
SENSORY MOTOR RETUNING AND CONSTRAINT THERAPIES
Constraint-induced (CI) movement therapy has shown promise as an effective intervention for rehabilitation following stroke and is considered a potential therapy for other motor disorders, including hand dystonia (18). In hand dystonia, the nondystonic finger(s) are immobilized by splinting, forcing the dystonic finger to work in concert with the other fingers. In one study, musicians were splinted to immobilize the nondystonic fingers and instructed to play their instruments for 1½ to 2½ hours per day for 8 consecutive days and then to use the splint for 1 hour per day for up to 1 year. In this study, the dystonia improved in pianists and guitarists, with modest benefit in wind instrument players (19). Associated with improvement in skills was a modification of the abnormal representation of the dystonic fingers in the somatosensory cortex (20). However, application of this technique to writer’s cramp showed only modest effects in 50% of subjects, and no changes in cortical excitability (21). A more complex, intensive program of sensorimotor retuning with and without biofeedback demonstrated improvement in occupational hand dystonia in an open-label, prospective study (22,23), but controlled studies of this technique have not yet been completed.
Conversely, other investigators have applied the opposite technique in which the dystonic arm is immobilized by splinting for periods of 4 to 5 weeks, followed by a period of retraining of the dystonic arm. This technique was used in an open-label study of eight patients with occupational hand dystonia. Following the splinting period, all patients had initial clumsiness and weakness that recovered in approximately 4 weeks with improvement in dystonia that was sustained for up to 24 weeks in seven patients (24). This was followed by a larger study that included 19 patients with either writer’s cramp or musician’s dystonia. This study found variable outcomes, with 20% reporting no benefit and approximately 45% reporting moderate-to-marked benefit (25). A single study noted that a combination of constraint therapy with retraining may improve musicians hand dystonia for up to 1 year (26). A controlled study has not yet confirmed these findings. In contrast, dystonia may also arise from prolonged immobilization with casting (27).
Some patients with dystonia have sensory abnormalities with deficits in spatial discrimination (28,29). Based on this observation and others, sensory training (e.g., reading Braille) has been proposed as a treatment for hand dystonia. In one study, 10 patients with hand dystonia were trained to read Braille and demonstrated improvements in both sensory perception and hand posturing (30). With ongoing training, improvements were sustained over 1 year. However, benefit was reduced or lost if practice was discontinued (31).
PHARMACOLOGIC TREATMENTS: ORAL MEDICATIONS (Table 29.1)
ANTICHOLINERGIC AGENTS
Within the striatum, there are giant aspiny interneurons that release acetylcholine. These constitute up to 3% of the striatal neuronal population. These interneurons have extensive connections within the striatum acting via nicotinic and muscarinic (M1-like and M2-like) cholinergic receptors, and have varied actions depending on the specific cholinergic receptors with which they interact. Although there is little evidence that cholinergic overactivity is involved in the pathophysiologic mechanisms of dystonia, indirect evidence, including the effect of anticholinergic agents and elevation of cholinergic tone in DYT1 rodent models, suggests that this may be on factor (32). Prior to the availability of BoNT, anticholinergic drugs were the primary treatment for dystonia. In one of the first systematic evaluations of anticholinergic agents, an open-label observational study in 23 children and 52 adults showed that 61% of children (<18 years) had a moderate-to-dramatic response with high doses of trihexyphenidyl (average dose 41 mg) and 38% of adults benefitted. The children had fewer adverse effects than the adults (33). Similarly, a retrospective study of 358 patients with dystonia found that 43% had a good response, with the greatest benefit in younger patients with generalized dystonia (34). An open-label trial assessing escalating doses of trihexyphenidyl showed that 37% had moderate-to-marked improvement at a mean dose of 21.5 mg. The patients who benefitted were younger, with a shorter duration of dystonia and were able to tolerate higher doses (35). There have been two prospective placebo-controlled studies. In one study, trihexyphenidyl at doses from 5 to 120 mg per day was evaluated using a crossover design and 18 week treatment duration in 31 young (9–32 years) patients with predominantly generalized dystonia. This study showed that 71% of patients improved, with 42% reporting substantial benefit after a follow-up of 2½ years (36). A second study in cranial dystonia assessed trihexyphedyl up to 12 mg in cranial dystonia compared to placebo and a peripheral anticholinergic. This study found only rare improvement using trihexyphenidyl, with many patients discontinuing due to unacceptable side effects (37). A more recent study compared trihexyphenidyl to BoNT injections without a placebo arm. At the end of the study, trihexyphenidyl at a mean dose of 16 mg did not improve dystonia symptoms, and more than half of the patients reported worsening with frequent adverse effects (38). There are other agents with anticholinergic properties that may be better tolerated, including ethopropazine, procyclidine, and biperidin, but no comparative studies have been done.
| Oral Medications Useful for the Treatment of Dystonia |
Pharmacologic Agent | Efficacy and Comment (Level of Evidence) | Side Effects |
Anticholinergic/antihistaminic Trihexyphenidyl (Artane) Benztropine (Cogentin) Procyclidine (Kemadrin) Diphenhydramine (Benedryl) Ethopropazine (Parsidol) | Effective in approximately 40% of young-onset, generalized dystonia (Class I) May be effective in focal and segmental dystonia (Class II, III, IV) Benefit limited by side effects Requires slow upward titration | Dry mouth; may lead to dental caries Blurred vision Exacerbation of acute-angle glaucoma Urinary retention Memory problems Sedation Confusion Hallucinations Heat intolerance |
Baclofen (Lioresal) | Effective in approximately 20% (Class III and IV) High doses tolerated in children (Class III) Benefits limited by side effects; intrathecal baclofen minimally successful | Nausea Sedation Dysphoria Muscle weakness (in those with spasticity-associated) Withdrawal effects on sudden discontinuation |
Clonazepam (Klonipin) | Effective in approximately 15% (Class IV) Possibility for addiction Withdrawal effects on sudden discontinuation | Sedation Depression Confusion Dependence |
Dopamine agonists Carbidopa/levodopa (Sinemet) | Dramatic response in the dopa-responsive form of dystonia Effective in 12% of primary dystonia (Class IV) Test for levodopa response in young-onset generalized and focal dystonia to rule out dopamine-responsive dystonia | Nausea (especially at initiation of therapy) May worsen dystonia Rapid discontinuation possible |
Muscle relaxants Tizanidine (Zanaflex) Cyclobenzaprine (Flexoril) | Limited benefit in some patients Side effects frequent (Class IV) | Sedation Dysphoria |
Antiepileptic medications Carbamazepine (Tegretol) Gabapentin (Neurontin) | Benefit in <10% (Class IV) | Ataxia Sedation |
Dopamine depleting agents Tetrabenazine | Benefit for dystonia (Class IV) Not available in the United States; available from Europe/Canada Requires a very slow upward titration | Depression Dysphoria Parkinsonism |
Dopamine antagonists (antipsychotics) Atypical Clozapine (Clozaril) Quetiapine (Seroquel) Typical Pimozide Haloperidol | Effective in up to 25% (Class IV) Clozapine requires weekly blood counts and may cause life-threatening agranulocytosis | Atypical antipsychotics: Sedation, orthostatic hypotension, metabolic syndrome, sudden death, seizures Clozapine: agranulocytosis (requires monitoring). Typical antipsychotics: Possibility of tardive dyskinesia and the other adverse effects from this class of medications severely limits usefulness. Not recommended for dystonia |
One of the greatest limitations to the use of anticholinergic drugs is the frequent occurrence of adverse effects. Adverse effects are not correlated with serum levels, but rather increase with increasing age (33,39). Peripheral side effects, such as dry mouth, blurred vision, and urinary retention, may be reversed using a peripheral cholinesterase inhibitor (glycopyrrolate). The central side effects are dose-limiting, and include memory loss, confusion and sedation, psychiatric disorders, chorea, and insomnia. If side effects occur with one anticholinergic agent, it may be possible to change to another agent. If side effects occur in the absence of benefit, anticholinergic treatment should be discontinued. Discontinuation of anticholinergic agents should be done gradually, as withdrawal effects may occur (33).
DOPAMINERGIC DRUGS
The use of levodopa in dopa-responsive dystonia is essentially an etiologic treatment and, in low dose, rapidly leads to a complete normalization of symptoms. In other forms of primary dystonia, carbidopa/levodopa and other dopamine agonist drugs can be useful, but have not been sufficiently evaluated. Most evidence is class III to IV. Early observations were inconsistent, with some open-label studies suggesting benefit (40,41) and others reporting no improvement or worsening (42,43). In a retrospective study, approximately 12% of 41 dystonic patients treated with dopamine agonists were found to have a good response (34). Direct dopamine agonists, including bromocriptine, lisuride, and apomorphine, have not been promising (44). Although there is insufficient evidence to support the use of levodopa for primary dystonia, the tolerability of the drug, the rapidity of a test trial, and the expanding phenotype of dopa-responsive dystonia (45–47) support an empiric trial in patients with primary generalized and focal dystonia (48). Levodopa in combination with carbidopa is started at low doses (50–100 mg per day) and increased gradually to a maximum dose of 600 to 1,000 mg. The most frequent adverse effect of levodopa is nausea, particularly at initiation of therapy that can be treated with supplemental carbidopa. If there is no improvement with levodopa dose at 600 mg for 4 weeks, the drug can be stopped.
DOPAMINE-RECEPTOR ANTAGONISTS AND DOPAMINE DEPLETION
Dopamine-receptor antagonists (antipsychotic agents) have not been adequately studied as a treatment for dystonia. Open-label studies have shown good response in 35% of 26 patients treated chronically (34). Combination therapy with pimozide, anticholinergic agent, and tetrabenazine was suggested to be useful in severe disabling axial or generalized dystonia in open-label observational studies (49). Other typical dopamine antagonists, including haloperidol and phenothiazines, have anecdotally been observed to improve dystonia (44), but controlled trials are lacking. Side effects of the typical antipsychotics include reversible drug-induced parkinsonism and potentially permanent tardive dyskinesia. Atypical antipsychotics, including clozapine and quetiapine may be useful in generalized dystonia, craniofacial dystonias, and oromandibular dystonia, although results have not been consistent in the small number of open-label studies conducted (50–54). The side effects of clozapine include sedation and agranulocytosis requiring frequent blood monitoring. Quetiapine does not require hematologic monitoring. There are only case reports of its effect in primary dystonia (55). Side effects of the atypical antipsychotics include sedation, orthostatic hypotension, lowered seizure threshold, and metabolic syndrome (diabetes, dyslipidemia, and hypertension, with associated obesity) (56).
Tetrabenazine depletes monoamines, including dopamine, through its action as an inhibitor of vesicular monoamine transporter 2 (VMAT2), and has additional dopamine-receptor antagonist properties (57). Early case series and retrospective chart reviews found benefit with tetrabenazine, alone or in combination with other agents in 25% of dystonia patients (34,49,58,59). One randomized, placebo-controlled study showed improvement in dystonia in four of six with cranial dystonia (Meige syndrome), and five of six patients with other forms of dystonia (60). A retrospective chart study showed improvement in 64% of 108 idiopathic dystonia patients (58,61). A more recent retrospective chart review in 132 dystonia patients reported that 67% had a marked or moderate reduction in movements with 50 to 75 mg of tetrabenazine. The improvement was sustained over approximately 3 years of follow-up in the 44% of patients who continued the drug (62). Side effects are common and include sedation, depression, parkinsonism, akathisia, nervousness, and insomnia (57,62). Tetrabenazine is approved in the United States for treatment of chorea in Huntington’s disease, but is more difficult to obtain for patients with dystonia.
BENZODIAZEPINE DRUGS: CLONAZEPAM
Clonazepam is a benzodiazepine that is used frequently for dystonia but has not been evaluated in controlled studies. Clonazepam was observed to be beneficial in retrospective studies for 21% of cervical dystonia (CD) patients at doses ranging from 1.5 to 12 mg per day (34). This drug may be particularly useful to treat CD with predominant dystonic head tremor (63,64). Small case series have observed benefit for blepharospasm (65). Modest benefit for dystonia has been reported with intravenous clonazepam (66). Benzodiazepines may be particularly useful in patients with myoclonic dystonia (67). The usual starting dose is 0.5 mg in the evening. Clonazepam is gradually escalated to benefit or side effects. Doses up to 8 mg can be used. The adverse effects include sedation, depression, confusion, and dependence.
BACLOFEN
Baclofen, a presynaptic GABA B receptor agonist, has been administered by both oral and intrathecal routes for the treatment of dystonia and spasticity. For dystonia, there are no controlled studies, and most reports are from retrospective chart studies or case series. In retrospective studies, approximately 20% of dystonia patients reported a good response (34). In a case series of children with primary generalized dystonia, baclofen when added to other agents at doses up to 120 mg, 7 of 16 patients had substantial or moderate improvement (68). A subsequent review showed that 14 of 31 children with dystonia improved using a dose range from 40 to 180 mg per day (69). Adults did not fare as well. For cranial dystonia and blepharospasm, initial benefit was seen in 28 of 60 patients; however, only 11 continued on baclofen with the primary reason for discontinuation being side effects (69). Patients with cervical dystonia were the least likely to benefit, with only 11% obtaining a good response at a dose range of 25 to 200 mg per day (69). Baclofen is initiated with small doses of 5 mg three times a day. Dose escalation is done slowly, allowing a week between dose increases. The most common side effects from baclofen are dizziness, sedation, nausea, and urinary symptoms. Confusion, hallucinations, and paranoia have been reported, but are rare. Sudden withdrawal of baclofen may precipitate psychosis or seizures or dramatic increase in dystonia.
In contrast to oral administration, intrathecal administration of baclofen (ITB) using an infusion pump allows high spinal fluid levels with the highest concentration of baclofen in the lower thecal sac. Theoretically, this method of treatment could reduce the occurrence of central side effects of baclofen. ITB has been shown to be effective for the treatment of spasticity and spasticity associated with dystonia (70). Its usefulness as a treatment for primary generalized or focal dystonia has yet to be established, and there are conflicting results from case reports (71–75). When administered in the high cervical spine or into the cerebral ventricles, there may be benefit (76). Intrathecal baclofen has numerous potentially serious complications, including surgical complications and malfunction of the pump causing sudden withdrawal (77,78). ITB is not currently viewed as a treatment option for primary dystonia.
OTHER TREATMENTS
Local injections of lidocaine, an anesthetic drug, improve symptoms of focal dystonia transiently (79–81). Mexiletine is a lidocaine derivative that is used to treat cardiac ventricular arrhythmias. Mexiletine has been assessed in open-label trials for the treatment of cervical and generalized dystonia using oral doses of 450 to 1,200 mg per day (82–84). Although the results have been promising, with sustained improvement in dystonic symptoms, controlled trials have not yet been published. Mexiletine is well tolerated, with the most frequent side effects being upper gastrointestinal symptoms, dizziness, ataxia, and dysarthria.
Other treatments have been reported anecdotally. Muscle relaxants such as tizanidine, cyclobenzaprine, orphenadrine, and methocarbamol have been used for dystonia, but evidence for improvement is lacking (85). One controlled study in a small number of patients found that nabilone, a cannabinoid receptor agonist, did not reduce dystonic movements (86). Anticonvulsants, although beneficial for paroxysmal dyskinesia, have not been adequately studied in primary dystonia. A small open trial of riluzole demonstrated benefit in cervical dystonia (87).
BOTULINUM TOXIN
BoNT is a large complex protein produced by an anaerobic, spore-forming bacillus, Clostridium botulinum. C. botulinum is a ubiquitous bacterium widely dispersed in the environment. The spores are heat-resistant. In the appropriate environment (anaerobic conditions, low acidity, and water), the clostridial spores germinate into cells, reproduce, and produce a toxin complex. The toxin complex varies and includes associated proteins, both hemagglutinin and nonhemagglutinin components depending on the specific subtype (88,89). There are seven antigenic distinct serotypes of BoNT, A to G. Each serotype consists of subtypes that are genetically distinct having at least a 2.6% difference in amino acid sequences. These genomic distinctions may afford subtle differences in the activity of a subtype. For example, BoNTA has five subtypes that bind SNAP-25 (90), but subtype BoNT/A1 and 2 cleave SNAP25 more efficiently than the other subtypes, and BoNTA2 appears to have a more rapid translocation into cells (91). Currently, only serotype A and B are available for clinical use.
BoNT was first used in humans in the 1970s for the treatment of strabismus (92). Subsequently, the safety and efficacy of BoNT was established for blepharospasm and cervical dystonia through controlled clinical trials (93), with approval by the Food and Drug Administration for use in these dystonic indications. Although there is insufficient evidence due to the lack of controlled clinical studies, BoNT is also used in other focal dystonias, including spasmodic dysphonia, writer’s cramp, oromandibular dystonia, and limb dystonia.
Chemodenervation with BoNT is considered the treatment of choice for CD. BoNT injections as a treatment for CD was first described in the mid-1980s (94). Subsequently, multiple, large, controlled studies provided class A evidence for benefit (93,95–97). In the United States, there are four brands of BoNT that have received approval by the Food and Drug Administration for the indication of CD and are available for use in the United States. The BoNT formulations that are currently available include onabotulinumtoxinA (BOTOX®), abobotulinumtoxinA (Dysport®), incobotulinumtoxinA (Xeomin®), and rimabotulinumtoxinB (Myocbloc®). Each brand of BoNT, although formulated differently, has a similar overall mechanism of action.
Currently, there is no evidence to indicate that one brand or serotype is superior to another. It has been determined that patients who have developed secondary, antibody-mediated resistance to the effects of serotype A may benefit from serotype B (98). However, it is important to recognize that the dosing of serotype B should be based on the starting doses for that serotype, without use of any type of conversion factor. The lack of a dosing equivalency is also recognized for the different brands of serotype A. Comparative trials have shown variable results; hence, there is no established dosage equivalency among these brands. If a patient is currently being treated with one brand, the dose of another brand cannot be determined from previous doses. Rather, each brand should be regarded as a unique formulation, and administered at doses in accordance with those described in the package insert and the controlled trials of that particular brand.
The adverse effects arising from BoNT injections are usually related to spread of toxin from the injected muscle. The most frequent side effects include pain at the injection site, dysphagia, and neck weakness in cervical dystonia, sometimes associated with pain. Dysphagia is one of the most serious adverse effects. It occurs in approximately 30% of patients injected. The risks for dysphagia have not been adequately studied. The most widely cited factor is dosage and placement of the BoNT injections into anterior muscles, including the sternocleidomastoid, scalene complex, and digastrics muscles located under the chin. In particular, a summation of injections greater than 100 units of onabotulinumtoxinA or similar doses of the other brands into these anterior muscles or an injection into the lower two-third of the sternocleidomastoid muscle is thought to increase the chance for dysphagia (99). In addition, a small neck circumference may contribute to dysphagia, and some experts recommend using smaller doses in these patients. Typically, dysphagia is mild to moderate, occurring approximately 1 to 3 weeks after injection, with duration of 1 to 3 weeks (100). However, there have been patients with severe dysphagia requiring temporary nasogastric feeding. Neck weakness begins around 1 to 2 weeks after injection, and may present as neck pain that differs from the dystonic pain. Patients will note that they have difficulty lifting their head from the pillow, or a sense that the head is unstable or will droop when bending forward.
Because CD is a life-time condition, and BoNT effects are transient, patients will require repeated injections at approximately 3- to 4-month intervals. Some patients will never benefit from injections, and are called primary nonresponders. This occurs most frequently in patients with structural abnormalities, such as contractures, or those with predominant anterocollis, in which the muscles are not safely accessible for injection (101,102). A secondary nonresponse is defined as the loss of benefit after an initial good response to a series of prior injections (103). As there are many factors that can contribute to reduced benefit following a single injection, secondary nonresponse is applied to those patients who fail to benefit from at least two injection series. Secondary nonresponse can occur for a variety of reasons. Common causes for secondary nonresponse include injection of the wrong muscles, inadequate dosing, or unrealistic patient expectation. Repeated exposure to a foreign protein such as BoNT may give rise to the development of neutralizing antibodies. These antibodies may cause a secondary nonresponse to injection associated with an absence of atrophy of the injected muscles and lack of typical adverse effects. Although the factors that cause neutralizing antibodies are not clear, most recommend that BoNT be given at the longest possible intervals, at the lowest effective doses (104). However, with currently available BoNTA, the occurrence of immunoresistance appears to be low (105,106). It may be possible to provide treatment at a shorter interval (107), although this is currently under investigation. Whether BoNTB has similarly low antibody production is not known.
SURGICAL THERAPIES
When medical interventions fail, surgical approaches may be appropriate. Surgery for dystonia has included muscles, nerves, and the central nervous system. In cervical dystonia, rhizotomies, ramisectomies, peripheral nerve ablations, and myectomies have been tried. Of these, selective denervation with resection of posterior branches of C1 to C6 and ablation of the spinal accessory nerve (the Bertrand procedure (108)) has been used the most extensively. The patient outcomes from selective denervation have not been well studied, and vary markedly.
Central Nervous System Surgical Approaches
Initially, ablative surgeries were performed for dystonia, targeting nuclei in the thalamus bilaterally. The results of bilateral thalamotomies have had mixed results. One prospective study in 54 dystonia patients, 54% with secondary dystonia, showed improvement predominantly in the limbs in 59% of the patients (109). Bilateral thalamotomies were frequently complicated by dysphagia, dysarthria, and pseudobulbar palsy. Subsequently, ablations were targeted toward the globus pallidus interna (GPi). The results with this new target were superior to thalamotomy with fewer adverse effects (110). When DBS became available, ablative surgery was largely abandoned in the United States. Multiple studies evaluating the effects of DBS in GPi showed marked improvement in dystonic symptoms (111). In isolated (primary) generalized dystonia, multicenter studies demonstrated that GPi-DBS resulted improvement of 42% to 51% of patients (112,113). Recently, the subthalamic nucleus (STN) has been evaluated as a target site. One study compared stimulation of the GPi to the STN in 13 patients with generalized or focal dystonia. This study showed that STN stimulation may be superior to GPi. Side effects showed increased poststimulation depression with GPi, but increased hyperkinesias with STN stimulation (114). A retrospective study designed to evaluate the optimal area for electrode placement using the model of volume of tissue activation (VTA) in 21 DYT1 dystonia patients showed that there was a circumscribed area within the GPi that appeared to be the optimal target (115). Factors that have been reported as associated with the best clinical outcome following GPi stimulation include mobile primary dystonia, shorter disease duration, and younger age at onset. However, these factors remain controversial (116). GPi-DBS has been used successfully in cervical dystonia, cranial dystonia, and upper limb dystonia (111).
GENERAL PRINCIPLES FOR TREATMENT OF DYSTONIA
GENERALIZED DYSTONIA (Fig. 29.1)
In generalized primary dystonia, oral medications may be very useful and are often the first line of treatment. Levodopa in combination with a dopa decarboxylase inhibitor such as carbidopa administered in doses up to 1,000 mg is recommended as the first drug. Marked response to levodopa suggests a diagnosis of dopa-responsive dystonia, and other treatments will not be necessary. Failing levodopa, anticholinergic agents are often used as the oral agent. Anticholinergic agents can be very effective at high doses and are well tolerated in children. If there is inadequate benefit from anticholinergic agents alone, then combination therapy with the addition of tetrabenazine (if available), baclofen, or clonazepam may be useful.
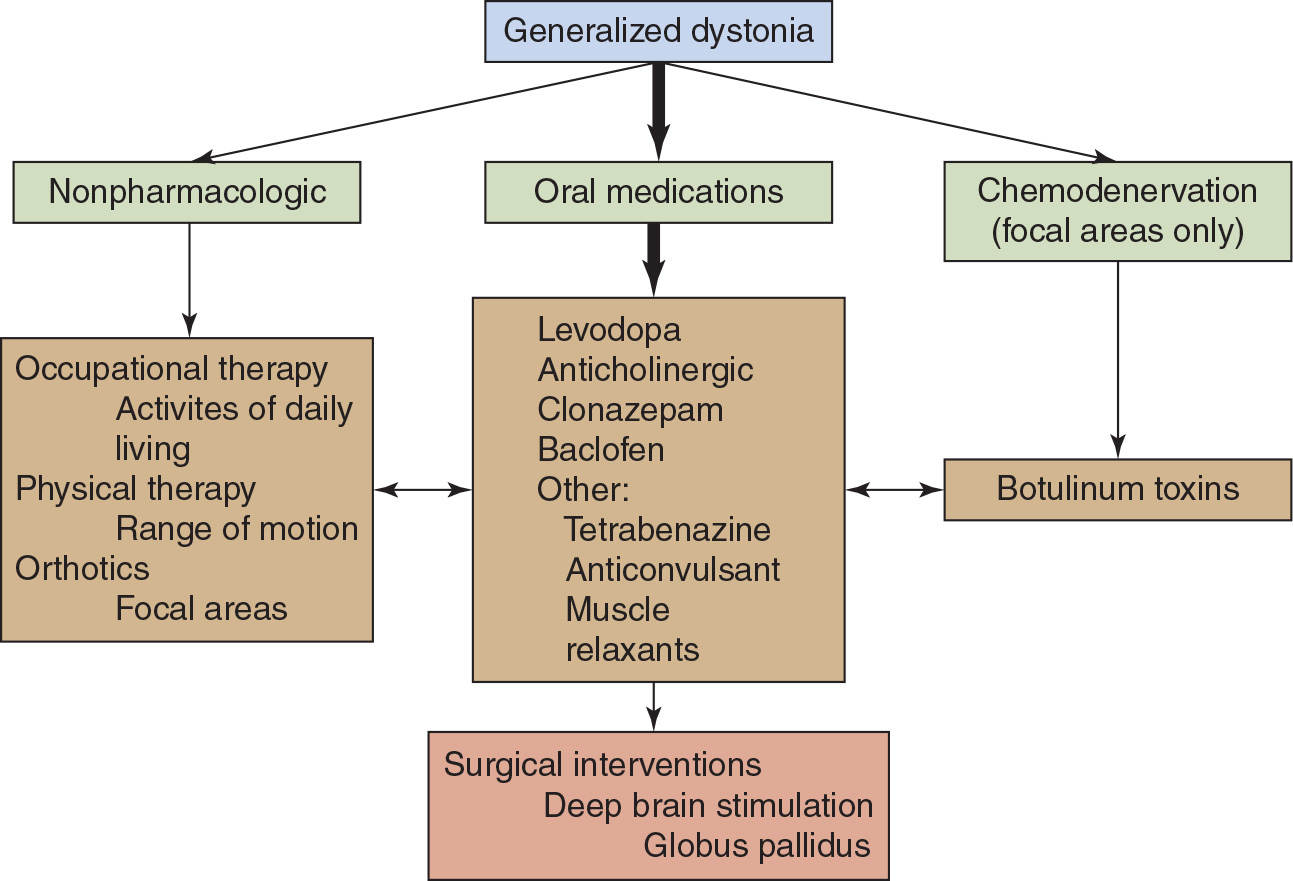
Figure 29.1. Guidelines for treatment of generalized dystonia. The thick arrow indicates the primary treatment option. The therapies indicated should be modified to the specific patient.








