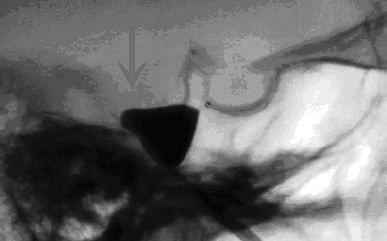
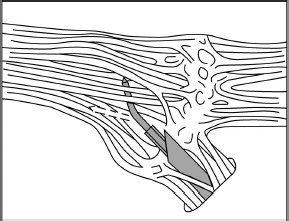
Chapter 19 Case A 65-year-old woman had typical V2-V3 trigeminal neuralgia that became unresponsive to medical treatment. Magnetic resonance imaging of the head is normal, and the patient is in good health. Participants Balloon Compression Rhizotomy for Trigeminal Neuralgia: Jeffrey A. Brown Microvascular Decompression for Trigeminal Neuralgia: Peter J. Jannetta Radiofrequency Rhizotomy for Trigeminal Neuralgia: G. Robert Nugent Stereotactic Radiosurgery for Trigeminal Neuralgia: Stephen J. Monteith and Jason P. Sheehan Moderator: Treatment of Patientts with Trigeminal Neuralgia: G. Robert Nugent More than a dozen years have passed since the first volume of Controversies in Neurosurgery raised the issue of treatment of trigeminal neuralgia (TN). At the time, I recommended percutaneous trigeminal nerve balloon compression. But now I suggest that treatment depends on a number of factors. In this section, I outline why and for which circumstances I recommend this procedure. I also explain the circumstances for which I do not recommend this procedure as my first choice. I co-chair the TNA–Facial Pain Association (formerly the Trigeminal Neuralgia Association), which serves as an advocate for patients with TN and related facial pain conditions by providing information, encouraging research, and offering support. My mission is to serve as a balanced source of information for each patient, tailored to that patient’s personal needs. In this role, I am not an advocate of any single surgical treatment for TN. Ideally, a neurosurgeon with knowledge of a wide range of medical and surgical options should evaluate the patient described in this chapter’s case presentation. Must medical management always precede any surgical option? When can we conclude that medical management has failed? One should increase the dose of carbamazepine when it is used to treat facial pain, for example, until pain relief or an average maximum maintenance dose of 600 mg daily is achieved. Too rapid an increase can cause lethargy, leading patients to assume that they have a drug intolerance. Nearly all patients taking carbamazepine report that it reduces their ability to concentrate, to think, and to react.1 Doses of carbamazepine greater than 600 mg per day increase the risk of bone marrow suppression in addition to cognitive depression. Patients taking other anticonvulsant medications may reach tolerance levels because of metabolic issues. High-dose treatment with oxcarbazepine often causes hyponatremia. I do not recommend multiple drug regimens. When a single anticonvulsant drug ceases to be effective in alleviating classic TN pain, it is unlikely that additional drugs will succeed without causing cognitive deficits. Furthermore, some patients may not tolerate any anticonvulsant therapy and need to proceed to surgical treatment. Is a 65-year-old woman too old to undergo microvascular decompression (MVD)? Current thinking suggests evaluating the relative medical condition of the patient rather than the absolute age. For example, a 63-year-old woman with a previous stroke associated with atrial fibrillation who is also on chronic anticoagulation therapy is at significant risk if she undergoes an MVD, or even balloon compression. The mean age at treatment in my series of patients who underwent balloon compression is 68 years. Anticoagulation is a significant issue in patients prone to TN. Balloon compression, radiofrequency thermal rhizotomy, and MVD require a temporary discontinuation of anticoagulation. Other comorbidities may increase the risk of MVD. However, patients in their seventh decade of life may still be reasonable candidates for MVD, especially given their overall greater longevity. The advantage of MVD is that the likelihood of recurrence requiring another surgical procedure and the risk of associated postoperative numbness are lower than those seen with ablative procedures. Older patients have larger subarachnoid spaces and require less cerebellar retraction to expose the trigeminal nerve. Technically, these circumstances simplify the surgery. However, the increased comorbidity in older patients may increase the morbidity of MVD in older patients. Because of the reduced compliance of the brain in older patients, there may be an increased risk of postoperative subdural hemorrhage and headache from pneumocephalus. No surgical series has shown these outcomes, however.2,3 Indeed, the evidence suggests that there is no increased morbidity in elderly patients undergoing MVD. One of the advantages of balloon compression over radiosurgery, with either the CyberKnife or the gamma knife, is that, in most situations, the pain is relieved immediately after surgery. In some situations, when compression is mild and the pain severe, the pain is not relieved for several days. With MVD, pain relief is also immediate, although there is no clear explanation for that immediate relief. Biopsies of the trigeminal nerve from the site adjacent to the compressive vessel show demyelinization.4 How this causes pain, and how removing a source of pulsating compression leads immediately to pain relief, is unknown. Is there an economic advantage to performing a percutaneous procedure? Balloon compression is an outpatient or overnight-stay procedure, whereas the length of stay after MVD is about 3 days. The length of stay is the most significant economic force increasing health care costs. If one assumes only a single procedure, then balloon compression is significantly less expensive. The recurrence risk with balloon compression, however, is 25% in 3 to 5 years. For MVD, the risk is 15% after 15 years. If one assumes an additional 25% recurrence rate every 5 years with balloon compression, and that such recurrence leads to a second surgery, and that the cost of MVD is three times more than that of balloon compression (because of the length of stay), then the two procedures are approximately equal in total cost after 15 years. Other factors, such as the cost of additional medications needed after recurrence, might further equalize the costs involved. The economic analysis is more complex than this, however. The present-day cost of a single operation may be less than the future costs of repeated balloon compressions. This point only serves to equalize the overall costs of the two operations. There is a flaw in this logic, however. Balloon compression also has an associated mortality rate and a risk of major disability. The risk is lower than with MVD, but present nonetheless. There have been deaths associated with balloon compression. What about the numbness associated with balloon compression? The incidence of bothersome numbness after compression in my series of patients is 6%.5 MVD can cause numbness, for example, because of nerve manipulation or heat transfer during bipolar coagulation of a vein. In the informed consent process, patients are essentially asked to weigh the benefit from the reduced risk of major morbidity or mortality of balloon compression against the discomfort that results from persistent facial numbness, even though mild. This is a form of behavioral economic decision making. Patients are weighing the value of initial pain relief, without the additional pain and risk of MVD, against their future risks of recurrent pain or need for surgery. In choosing between balloon compression and MVD, as “altruistic” neurosurgeons, we are evaluating personal, emotional, economic, immediate, and future health concerns.6 Several scenarios would lead me to recommend balloon compression for this patient as my first choice. I carry out thin-cut magnetic resonance imaging (MRI) scans in my office as a part of the patient’s initial evaluation. The absence of an obvious vessel associated with the trigeminal nerve may lead me to recommend an ablative procedure. If the patient’s pain is unremitting and he or she is unable to wait for the effects of radiosurgery, then I would recommend balloon compression. Pain relief is usually immediate. If the pain is in the first division, then balloon compression may be safer than thermal rhizotomy. Balloon compression selectively preserves the unmyelinated fibers that mediate the corneal reflex, which limits the likelihood of corneal anesthesia and visual loss when compared with thermal rhizotomy.7 If the patient, because of cognitive issues, cannot cooperate with the surgeon to evaluate the site of stimulation or the extent of sensory loss intraoperatively, as is done with thermal rhizotomy, then balloon compression is easier. In general, balloon compression is easier to perform than thermal rhizotomy because it does not require the patient to be awake during the procedure. If the patient has already undergone MVD and reexploration, then I may recommend balloon compression as the next procedure. Reexploration after MVD is reasonable, especially if there is a vascular association shown on thin-cut MRI scans. Patients may not be able to tolerate the thought of another decompression. Balloon compression is reasonable in such a situation. The technique of percutaneous balloon compression has been well described in previous publications5 (Fig. 19.1). An important issue to consider is the necessity of continuing anticoagulation. Warfarin, clopidogrel, and aspirin must be stopped before the patient undergoes balloon compression, and anticoagulation is restarted soon after surgery. Determining when to restart anticoagulation is based on how many times it was necessary to reposition the cannula or balloon during surgery and whether there is blood seen on postoperative computed tomography (CT) images. Are there any situations in which balloon compression is contraindicated? The patient with multiple sclerosis and bilateral TN presents some difficulty. Here, the function of the contralateral pterygoid muscle is important. Bilateral pterygoid muscle weakness will make it difficult for the patient to chew, but this weakness usually resolves within a month. Some patients cannot tolerate any lip or tongue numbness. For example, a professional musician who plays a woodwind or brass instrument could have difficulty with such sensory loss. More important, however, is the safety of discontinuing anticoagulation. Fig. 19.1 The lateral image intensifier view shows the correct “pear shape” (arrow) of the balloon when properly inflated with its tip positioned at the entrance to Meckel’s cave. If this pear shape does not appear, there will not likely be adequate compression pressure to injure the large myelinated fibers of the trigeminal nerve. Before surgeons found a way to apply the binocular surgical microscope to the operative treatment of TN, the treatment consisted of deliberate injury to the trigeminal nerve. Historically, the nerve was sectioned or the peripheral branches or ganglion injected with absolute alcohol. The object of the therapy was to replace one symptom, pain, with another, numbness. In the winter of 1965–1966, I developed a microsurgical procedure based on a clarification of the trigeminal nerve anatomy partly described by Dandy. At the first operation, performed with the assistance of John Alksne, the superior cerebellar artery was seen to pulsate into and compress the nerve. I said, “That’s the cause of the trigeminal neuralgia.” My lack of understanding of the process of acceptance was naive. But young and naive people see and do new things, partly because they do not understand that something cannot be done. In The Structure of Scientific Revolutions, Thomas Kuhn8 clearly organized the sequence of thoughts in the trials and assumption of new ideas. He was the first to describe the paradigm shift, and he clarified the sequence elegantly. His book should be read by anyone doing new work in science or medicine. It generally takes 20 years or more for a new paradigm to be developed and accepted. This time lag occurs as we wait for the old guard, to whom the new ideas are anathema, to retire or die. The younger generation accepts the new paradigm as more profound and acceptable than the old system of thought. But the better the new idea, the longer it may take for acceptance. The concept of vascular compression of the cranial nerves and of treatment with MVD went through this annealing process before general acceptance. It can now be safely said that, in many or most centers throughout the world, MVD is the treatment of choice for TN. And yet, several areas of controversy remain. The following questions are, in a sense, secondary and perhaps problem-solving areas, or “normal science” as Kuhn described it: Is TN always caused by blood vessels? Almost always. The exception is that many patients with TN due to multiple sclerosis have a causative blood vessel in addition to the sclerotic plaque in the root entry zone. The major question is not, “Is there a causative vessel here or not?” but rather, “Am I good enough to find all the causative vessels?” Can the offending blood vessels be seen on a scan? We obtain MRI scans not to see the offending blood vessel(s) but to rule out congenital abnormalities such as a Chiari I malformation, to find arteriovenous malformations, to see arterial aneurysms, and to identify dolichoectatic arteries. Even with advanced scanning techniques, the offending vessel is often missed. We frequently see a larger artery apparently compressing the nerve but find that a smaller artery, arteriole, vein, or venule is the true cause. It is less than smart to think that a blood vessel is not present and causing TN just because one cannot see it on MRI. The eye is a much better monitor than a scan. Is the size of the blood vessel important? No, no, and no. Are there subtle vascular compressions in TN that the surgeon should understand? Yes. An artery or vein under the ala of the cerebellum can be the only vessel or can contribute to the TN. Cerebellar support by a retractor can move a blood vessel off the nerve, as can the drainage of spinal fluid. I have seen four patients with a large vein inside the portio major, completely unexpectedly. A distal crossing vein, which may be hidden under a bony enostosis in dolichocephalic patients, may be missed. I occasionally see patients who have had a “negative exploration” at a prior operation have this vein. Should neurophysiological monitoring be performed intraoperatively? I use it. Although this operation can be done without it, I prefer it and not just for teaching purposes. The use of auditory evoked potential monitoring helps ordinary people like me to perform the MVD with a clean and safe technique. MVD without monitoring may be admirable to some, but I do not admire it. To extrapolate this thinking, one can do a vascular decompression of a nerve without magnification but not very well. Surgical progress implies that the difficult becomes common and the extraordinary, ordinary. What local care is necessary postoperatively? I do not send patients to the intensive care unit. It is unnecessary. The patients are comfortable in a bed on a regular ward and are ready for discharge the next day. I hardly ever have to readmit a patient to the hospital. Are there age restrictions regarding eligibility for MVD? In general, no. Young children are usually misdiagnosed, even by pediatric neurosurgeons. Our youngest patient suffered the onset of typical TN at age 7 months. We saw him at age 22 months, undernourished, undersized, underdiagnosed, and being fed through a gastrostomy tube. Elderly patients generally tolerate MVD well. The rule is, Do they have a 5-year estimated survival? Can they well tolerate 2 hours of general anesthesia? It is not chronology but general health that helps us make the decision regarding MVD. What TN syndromes can be treated with MVD? Typical TN is considered below. Here I discuss the variations. TN can become “atypical” for several reasons. The first is medication. Carbamazepine, the best of the anti-TN drugs, may relieve lancinating pain, but a background continuous pain, usually burning in character, may persist. More medication may stop the persistent pain. Other drugs may also do this but much less frequently. TN of long duration may convert from typical lancinating pain to constant burning pain (modified TN). Those patients who never had lancinating pain do not fare as well after MVD, and nothing else helps them. We can relieve the pain in 40 or 50% of these patients. Is there a clinicopathological relationship in TN? Yes. But it is not as clear as in the facial nerve (hemifacial spasm) or the auditory nerve (vertigo, disequilibrium, tinnitus, and hearing loss). In general, the clinicopathological relationships in TN are as follows: • Rostral compression, most frequently by the superior cerebellar artery, causes V3 TN. • Lateral compression, by a downward and upward looping superior cerebellar artery or by a bridging vein, most likely an aberrant trigeminal vein, especially in young women, causes V2 pain. • Caudal compression, most frequently in elderly men who are heavy cigarette smokers, causes isolated V1 pain. An advancing length of a superior cerebellar artery loop seems to be responsible for the spread of pain up the face from V3 to include V2 and all divisions. • Distal compression of the motor-proprioceptive fascicles, usually by the superior cerebellar artery, causes constant pain, usually burning in character. As this portion of the nerve is stretched by the artery, hyperactive autonomic dysfunction may frequently occur. Over an extended period, mild numbness becomes more prominent in the area of pain. The technique and results of a retromastoid craniectomy and MVD have been well described,9,10 and the article by McLaughlin and colleagues10 may be the best overview of these techniques. The retromastoid incision, 3 to 5 cm in length, must be placed posterior enough so that a good angle of sight into the cerebellopontine angle is achieved. The craniotomy is placed high and lateral at the junction of the transverse and sigmoid sinuses. The opening in the dura mater, either curvilinear or with a T into the corner, must allow exposure at the junction of the sinuses. The entire trigeminal nerve must be exposed and inspected. Both arteries and veins may move out of position away from the nerve because of the drainage of spinal fluid or retractor displacement of the cerebellum. The great majority of vascular blood vessels compressing the trigeminal nerve are overt and easily seen, whereas 5 to 10% are subtle. Many overt cases also have a secondary subtle cause, and these must always be appreciated. A vein or artery under the ala of the cerebellum, a very distal crossing vein, and a venule or arteriole must all be evaluated and treated. Pontine surface veins may be the cause of TN, more frequently in women than in men. Regrettably, these pontine surface veins are prone to recollateralizing after they have been coagulated and divided. This may occur as early as 6 weeks postoperatively. An early recurrence of TN is most likely due to the recollateralization of surface veins. Because the veins frequently lie under the pia mater instead of truly in the subarachnoid space, elevation and decompression using soft implants is tedious and difficult to perform without disrupting the integrity of the vessel wall. We try to save these veins in every case and this has reduced the early recurrence rate significantly. We do cranioplasties using titanium mesh secured in place with self-driving screws. This technique has decreased the incidence of chronic headaches, usually seen in 15 to 20% of patients with preoperative posterior fossa/cerebellopontine angle involvement, to virtually zero. How do patients react to MVD for TN postoperatively? If their TN is gone, they feel better than they did preoperatively just as soon as they are over the acute effects of anesthesia. They are usually ready for discharge on the first postoperative day. If they do not feel well, they are hospitalized for another day. If they develop a severe headache, a CT scan is done to rule out a blood clot or cerebellar swelling (very rare). Once the obstruction is cleared, a lumbar puncture is done with a small needle. Any pressure above 100 cm H2O is dropped halfway to 100. The patients are given one dose of dexamethasone (10 mg) parenterally. They almost universally feel well very rapidly. What are the principles of postoperative care? If patients have traveled to our center from a distant locale, we see them on the fifth postoperative day before allowing them to return home. Medications are decreased rapidly starting immediately after surgery, except for carbamazepine. To prevent withdrawal symptoms, this drug must be slowly decreased over a 10-day period after an initial decrease to a small maintenance dose of 600 to 800 mg per day. During the postoperative visit, we check for any hearing loss, most of which is mild, due to fluid in the middle ear, and accompanied by a sense of fullness in the ear. This may take 5 or 6 weeks to dissipate. The patients are told to call us if they have any problems, to let us know if they move or change their phone number, and to e-mail or send a card with a status report at Christmas. They are reminded that the trip home will probably tire them out and that this is normal. They are told that they can gradually increase their activity to normal over a 6-week period. They need to work up a sweat to the point of fatigue, and the best way to do this is by walking vigorously, a la Harry Truman. The pain of TN is so severe that many patients are satisfied with any treatment offered, provided pain relief is the outcome. The surgeon’s predilection and experience, however, often color the process of informed consent and predetermine the patient’s choice of therapeutic options. Naturally, a neurosurgeon experienced in microneurosugery of the posterior fossa might strongly recommend the surgically challenging MVD to the patient presented in this chapter. Currently, the MVD procedure is considered the treatment of choice for TN because it stops the pain without the trade-off of numbness in the face. The second option is one of the treatments aimed at inflicting some damage (at best minimal) to the ganglion or retrogasserian rootlets to eliminate the pain. These include radiofrequency (RF) thermocoagulation, glycerol infusion, or balloon compression. The third treatment option is the currently popular gamma knife radiosurgery procedure. Here I present a case in favor of RF treatment, which argues that most surgeons misuse this technique by making the face too numb, believing that this is necessary to ensure a good result. This is a self-defeating practice and often leads to the patient’s dissatisfaction because of annoying dysesthesias in the face. Many surgeons do not realize that only a minimal sensory deficit is necessary for lasting relief from the pain. Only the RF technique, when properly performed, permits control of both the location and extent of the deficit produced. The aim is to produce a mild degree of numbness in the face with which the patient can be comfortable. The key word here is comfortable. The indications for the RF procedure are similar to those for other procedures aimed at creating some injury to the trigeminal nerve. Patients must have true or classic TN because this procedure is definitely contraindicated in other, atypical types of facial pain. Differentiating TN from other types of pain should not be a problem because true TN is one of the classic syndromes in all of clinical medicine. Patients with true TN have pain that is usually refractory to medical management or they suffer from intolerable side effects of such therapy. Those who have had a satisfactory result from a peripheral block may opt for more permanent numbness. Furthermore, some patients will not choose an intracranial operation with its remote, but not insignificant, risk of complications. Finally, some patients simply opt for a more benign, less time-consuming, and less expensive form of treatment. The informed consent form for the RF treatment must specify the possibility of annoying dysesthesias in the face, the worst of which is anesthesia dolorosa; recurrence of the pain, requiring a second treatment; and the very remote and unlikely possibility of visual impairment from inadvertent corneal anesthesia. Anesthetic safeguards must be in place to prevent intracranial hemorrhage from the occasional reflex hypertensive bursts that may occur when the foramen ovale is penetrated. I have argued that the RF technique allows much more control of the extent and location of the lesion than other destructive techniques. This control is achieved through the use of a small, angled electrode of 0.4 mm (Fig. 19.2). Because lesions behind the gasserian ganglion are virtually painless, or at least the pain is tolerable, lesions with this small electrode can be made while the patient is awake. It is thermal lesions in the ganglion and distal divisions of the nerve that are intolerably painful. Of great importance, therefore, is creating the lesion with its sensory deficit while the patient is fully awake, permitting constant online monitoring of the sensory deficit being produced and at the same time protecting corneal sensation. By making small incremental lesions, it is possible to “creep up” on a moderate sensory deficit. I do not use the larger temperature monitoring electrode that is popular with many because its heat reaches the ganglion and the resulting pain requires that the patient be anesthetized. Furthermore, the required final temperature varies tremendously depending on where within the retrogasserian rootlets the electrode tip lies. The lesion develops at a lower temperature if the electrode lands ideally in those rootlets. Sweet and Wepsic11 note that “the necessary final electrode temperatures varied through an amazing range from 47° to 108°C,” and Siegfried12 states that the temperature at the tip of the electrode and the intensity of current show no consistent direct relationship. Relying on temperature may lead to a lesion that is too dense. Therefore, I am convinced that online monitoring of the awake patient while the lesion is being made is far more important than temperature monitoring. Fig. 19.2 This small electrode enables lesions to be made behind the ganglion where the thermocoagulation is painless. The electrode permits online evaluation of the location and the extent of sensory deficit while the lesion is being made. The angle of the electrode introduces another parameter for localization in the rootlets in addition to the mere depth of penetration.
Treatment of Patients with Trigeminal Neuralgia
Balloon Compression Rhizotomy for Trigeminal Neuralgia
Microvascular Decompression for Trigeminal Neuralgia
Radiofrequency Rhizotomy for Trigeminal Neuralgia
The Radiofrequency Technique: A Method
Preliminary Considerations
Treatment of Patients with Trigeminal Neuralgia
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree