Neuropathic
Upper motor neuron
Cerebral palsy
Spinocerebellar degeneration
Friedreich ataxia
Charcot–Marie–Tooth
Roussy–Levy
Syringomyelia
Spinal cord tumor
Spinal cord trauma
Lower motor neuron
Poliomyelitis and other viral myelitides
Traumatic
Spinal muscle atrophy
Werdnig–Hoffmann
Kugelberg–Welander
Dysautonomia
Myopathic
Arthrogryposis
Muscular dystrophy
Duchenne
Limb-girdle
Facioscapulohumeral
Fiber-type disproportion
Congenital hypotonia
Myotoniadystrophica
Cerebral palsy (CP) has an estimated incidence of two per 1000 of live births [2], with an incidence of scoliosis 15–28 % on clinical or radiological examination in a general CP population [3]. In an institutionalized CP population, Madigan and Wallace [4] found a 64 % incidence of scoliosis. Of the physiological classification of CP, spastic quadriplegic CP has the highest incidence of scoliosis [3, 4]. The risk of scoliosis correlates with the level of ambulatory ability of the patients graded by Gross Motor Functional Classification System (GMFCS). Children with mild gross motor function limitation (GMFCS levels I-II) have no higher risk of developing scoliosis than the general population. In children with limited motor function (GMFCS levels IV and V), the risk of developing clinically moderate or severe scoliosis is 50 % [3].
Lonstein and Akbarnia [5] classified scoliotic curves as a result of CP into two groups: Group I curves, which are double curves with thoracic and lumbar components (S-curves) that behave like idiopathic scoliotic curves with higher likelihood of preservation of ambulation ability, and Group II curves with more lumbar or thoracolumbar curves that extended into the sacrum with associated pelvic obliquity (C-curves). The long, sweeping, and collapsing curves are more typical of neuromuscular curves in patients who are wheelchair-dependent or bed-ridden. The apex of these curves centered at the thoracic (T2–T10) or thoracolumbar (T11–L1) and pointed to the right (Fig. 12.1). Among institutionalized CP population, Group I and II curves have the same incidence [4]; however, Group II curves form the majority (94 %) [6] of the patients with CP who required surgical intervention attributable to pelvic obliquity, poor coronal balance, and large magnitude of the curve.
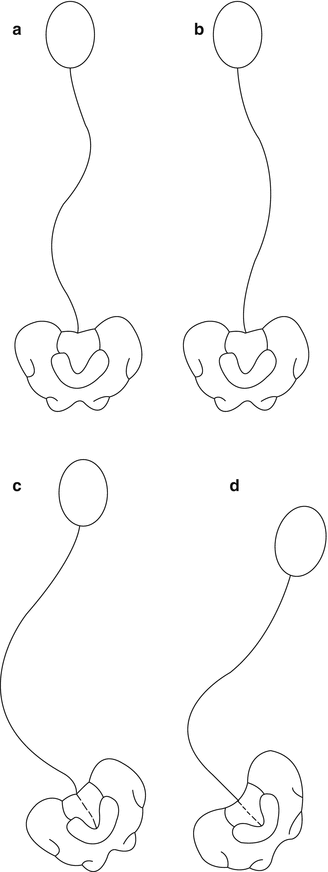

Fig. 12.1
Curve patterns in cerebral palsy scoliosis. Group I curves are double curves with little pelvic obliquity that may be balanced (a) or unbalanced (b). Group II curves (c, d) are large lumbar or thoracolumbar curves with marked pelvic obliquity (Adapted from Lonstein and Akbarnia [5], p 800)
12.2 Natural History
Although the age of onset can vary widely from 3 years old to 20 years old, neuromuscular scoliotic curves generally develop at a younger age than adolescent idiopathic scoliosis [7]. Some of the patients present with significant curves prior to the prepubescent growth spurt as a result of earlier curve onset. With the growth spurt, which is typical delayed, the flexible, postural curve tends to develop into a torsional structural deformity. Finally, a stiff curve of considerable magnitude develops before the growth complete.
The single most important factor that affects the magnitude of the curve is the severity of CP. There appears to be proportional relation between the severity of involvement of CP and the curve severity; 67 % of the quadriplegic and 18 % of the non-quadriplegic spastic CP in this study had curves that exceeded 60° [7].
While the rate of curve progression is highly variable, the average progression cited in one report is 0.8° a year in curves less than 50° and 1.4° a year in curves more than 50° [8]. During periods of rapid growth, much more severe progression can occur. Eighty-five percent of patients who had curve of more than 40° by age 15 years progressed to 60°, while only 13 % of those with a curve of less than 40° by age 15 progressed to 60° [7]. Some authors reported curve progression after intrathecal baclofen therapy was instituted to control severe spasticity even in skeletally mature patients [9]. In an adequately powered, case–control study, it was found that the rate of progression was not affected by the use of intrathecal baclofen therapy and that curve incidence and progression were rather related to neurologic involvement [10].
Curve progression increases the magnitude of deforming forces and leads to subsequent deformity, truncal imbalance, and pelvic decompensation. The pelvis is often the end vertebra – the most tilted vertebra with residual axial rotation of the C-curve. This was described as the pelvic vertebra by Dubousset. Less commonly, pelvic obliquity presents as a compensatory fractional curve to the C-curve. Pelvic obliquity alters the sitting position and the pressure at the typically well-distributed sitting tripod at both ischial tuberosities and pubic symphysis. The undue increased pressure at the ipsilateral ischial tuberosity is further exacerbated in patients who have increased pelvic tilt and results in pressure sores.
Depending on the dominant deforming forces and the interplay of spasticity, patients may present with kyphoscoliosis or lordoscoliosis. In kyphoscolosis, progressive deformity with associated pelvic obliquity and retroversion may compromise the often-limited ambulatory function particularly standing to transfer. Significant pelvic obliquity makes sitting adaptation difficult and sometimes impossible. In lordoscoliosis, patients may present with extensor posturing clinically. The progressive deformity renders sitting impossible. Patients may need to be nursed in a semi-reclined position in a wheelchair. This group of patients may present with acute pain that is not alleviated with any sitting adaptation.
Significant spinal deformity is known to compromise cardiopulmonary function, affect gastrointestinal motility, and result in rib-pelvis impingement. The morbidity associated with these are difficult to quantify in this vulnerable and low-demand population especially those with pre-existing difficulties in swallowing, dependence on G or J-tube, and multiple medical comorbidities and are unable to articulate their symptoms.
12.3 Clinical Presentation and Evaluation
Given the universal progressive nature of neuromuscular scoliosis, early diagnosis of the deformity is essential. Initial evaluation should consist of clinical monitoring by physical examination. During physical examination, the patient is examined in a sitting position for a curve and pelvic obliquity. When a curve is identified, the crux of the examination is to assess the flexibility of the curve clinically and the remaining growth potential by serial height and weight measurements and radiographic markers.
Curve flexibility is assessed by holding the patient up at the axillary areas in a sitting position. In a smaller framed patient, a clinical fulcrum bend test over the examiner’s knee is possible. Pelvic obliquity is assessed by lying the patient in a prone position with the hips and knees hanging free. Infrapelvic causes of pelvic obliquity such as hip subluxation/dislocation or adductors contracture are evaluated and managed appropriately. Suprapelvic causes of pelvic obliquity arise from the scoliosis and are assessed clinically for flexibility and reducibility.
When a significant curve is identified, standing position (when possible) 36-in. posterior–anterior (PA) and lateral radiographs of the spine should be obtained. Radiographs in sitting position may be obtained if the patient is unable to stand; it may be necessary to support the head and trunk in severely affected children with poor truncal control. At our center, we use a standardized sitting frame with lateral support straps to obtain films in the sitting position with minimal external support.
Radiographically, the curve characteristics (curve type, magnitude, and progression), spinal balance (sagittal and coronal), pelvic balance (pelvic obliquity and tilt), and the growth remaining indicators (status of the tri-radiate cartilage and Risser sign) are documented (see Fig. 12.2a, b). Vertebral rotation with rib deformity and wedging suggest that the deformity is structural rather than positional. Among the various techniques of pelvic obliquity measurement, horizontal pelvic obliquity has the least intra-observer and inter-observer variability [11]. The patient with established scoliosis due to CP requires at least yearly follow-up examination to assess curve progression, but with severe curves or during periods of rapid growth, biannual follow-up is desirable.
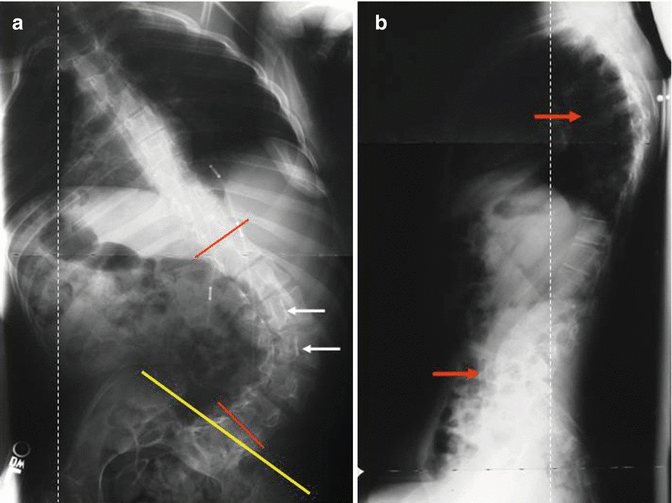

Fig. 12.2
When evaluating a patient with CP scoliosis, there are other radiographic parameters in addition to the Cobb angle (red lines). Note the severe apical rotation (white arrows), pelvic obliquity (yellow line) (a), hyperkyphosis, and hyperlordosis (red arrows) (b) and coronal and sagittal imbalance (dashed lines)
A magnetic resonance imaging (MRI) should be obtained if there is any suspicion of intraspinal pathology, such as very rapid progression at a young age, increasing lumbar hyperlordosis, or a change in neurologic status, which could be harbingers of a tethered cord.
12.4 Non-operative Care
In the global planning of disease management, several factors need to be considered. Of paramount importance are the alleviation of pain, preservation of function, and facilitation of daily care. Non-operative management of patients with neuromuscular spinal deformities should be directed at maximizing sitting ability and postural control to facilitate interaction with the surrounding environment. A normalized eyebrow-chin angle allows visual and cognitive stimulations with motor response.
Initial close observation of curves that are 20° or less is reasonable; if progression occurs, initial intervention with a brace may be an option. The role of bracing in CP scoliosis is dependent on the severity of the curve and neurological involvement. In a patient with spastic quadriplegic CP, it is generally accepted that bracing is ineffective but may slow the rate of progression. Miller et al. found no impact of a rigid thoracolumbosacral orthosis (TLSO) on scoliosis curve, shape, or rate of progression in spastic quadriplegic patients that were braced 23 h per day over a mean period of 67 months compared to a similar cohort that were not braced and were followed to spinal fusion [12]. Terjesen et al. [13] retrospectively examined a cohort of 86 patients with spastic quadriplegic CP and found a mean rate of progression per year of 4.2° with a custom-molded polypropylene TLSO. Interestingly, 25 % of the patients had no progression or progression of less than 1° per year. The degree of curve correction in the orthosis appeared to correlate with non-progression of the curve. Of note, Terjesen et al.’s study had a mean initial Cobb angle of 68.4°.
Although it may not alter the final disposition, a soft (polypropylene foam) TLSO can provide seating support and augment function. Improved sitting in a child may correlate with attentiveness in class, ease of care, improved self-image, and decreased rate of decubitus ulcers.
Another option for patients with flexible curves in need of seating support is the adjustment of offset lateral chest supports and modular seating systems on the wheelchair. This three-point control of the coronal deformity will prop up the child and address sitting balance. The wheelchair should be the primary seating device. In an ambulatory patient (GMFCS level I-II), it is believed that a hard brace may slow the progression of the curve similar to the patients with adolescent idiopathic scoliosis. The brace is indicated beyond 25° in immature patients with significant growth remaining. The brace should be worn for a minimum of 12 h. An optimal brace time is 16–18 h per day. Therapeutic stretching, electrical stimulation, or botulinum toxin is lacking scientific validity and should have no role in the management of deformity.
12.5 Rationale of Operative Care
Goldberg argued that the potential gains from interventions should be assessed by the following components: functional health gain, patient satisfaction, and technical success [14]. Given the wide spectrum of disease presentation and progression as well as the concomitant variability in functional status of the patient, the decision to proceed with operative correction and stabilization is based, in large part, on patient-specific factors with the broad aim of maintaining the functional health against the progressive deformity and its associated morbidity, achieving reasonable patient/caregiver satisfaction, and minimizing the complications associated with the surgical intervention.
For higher functioning patients, operative intervention aims to provide a more normal spinal balance and alter the progression of disease with the goal to preserve function with respect to ambulatory potential. The parents or caretakers can make an informed decision weighing the risks and benefits for their children.
For patients with no ambulatory potential (GMFCS 5), the aim is to maintain independence in sitting and facilitate care. As expected, the burden of care in this group of patients with severe learning disability may change significantly [15, 16]. As observed by Madigan and Wallace [4], the severity of scoliosis is directly proportional to the severity of involvement of CP. Concern has been raised regarding the risk of an extensive surgical procedure in a medically compromised patient. Surgical treatment in this group represents a palliative measure that allows the family to provide maximal medical treatment with the intent of caring for the child at home and keeping the child involved in school and other outside, community activities.
A prospective study by Larsson et al. in a cohort of neuromuscular scoliosis with a varied spectrum of learning disabilities found that the overall care burden decreased with improved sitting position and lung function (vital capacity) on follow-up [15]. Comstock et al. [17] assessed both patient and caregiver satisfaction in a cohort of 100 patients with total-body-involvement spastic CP who underwent spinal fusion. The satisfaction of both caregivers and patients was assessed via interview responses to standardized questions, and physical examination was used to assess functional status. Eighty-five percent of the parents interviewed indicated that they were satisfied with the results and would repeat the surgery again. There was an impression by caregivers that the patients had an improved self-image, and patients who were able to respond to questions confirmed this. Both parents and caregivers felt that the surgery had a positive impact on the patient’s sitting ability, physical appearance, comfort, and ease of care. Multiple authors including Bulman et al. [18], Sussman et al. [19], and Watanabe et al. [20] found similar satisfaction rates in their studies.
12.6 Indication for Surgery and Specific Considerations
In general, surgical intervention is considered for curve magnitude greater than 50° with significant deterioration in function [17, 22, 27]. There is sufficient evidence that these curves will progress, even if the child has completed his growth. For curves 60–90°, surgery is indicated when the deformity becomes stiff by physical examination, even if substantial growth remains. If the spine displays continued flexibility on physical examination during growth, surgery can be delayed until 90° and can still be performed with a posterior-only procedure. In a flexible curve of greater than 90°, sitting may be a challenge and further exacerbated by the associated pelvic obliquity.
In planning for surgery, specific considerations should be given to the level of instrumentation, early-onset scoliosis, sagittal plane deformity correction, pelvic and infrapelvic coronal deformities, intraoperative neuromonitoring, the necessity of anterior release, intraoperative femoral traction, and intrathecal baclofen therapy.
12.6.1 Level of Instrumentation
Patients with neuromuscular scoliosis are traditionally fused long, typically from T1/T2 to the sacrum including pelvic fixation. An increased incidence of proximal curve progression, especially proximal junctional kyphosis, has been observed if the cephalad level of instrumentation does not extend to at least T2 [21], since most of these children lack sufficient head control. In patients with pathological thoracic kyphosis, it may be necessary to extend the instrumentation to C7 for adequate thoracic kyphosis control.
Historically, there has been debate regarding when to extend the posterior spinal fusion to the pelvis. Pelvic obliquity has been noted to progress in neuromuscular scoliosis if the pelvis is not fused [8, 22, 23]. Many authors have recommended fusion to the pelvis in nonambulatory patients. In the ambulatory patient with pelvic obliquity, fusion to the pelvis has been traditionally avoided due to the belief that it will adversely affect ambulatory function [3, 24]. At our institution, a retrospective study by Tsirikos et al. [25] demonstrated preserved ambulatory function in ambulatory patients with CP that were fused with unit rod instrumentation, documented by gait analysis.
A subset of patients may be instrumented to L5 particularly if they use the gluteus maximus to propel their gait due to weak gastrocnemius. Significant pelvic rotation is expected clinically during ambulation. McCall and Hayes [26] retrospectively examined a cohort of patients with neuromuscular scoliosis in whom those with a stable lumbosacral articulation were instrumented with a “U-rod” (unit rod without the pelvic limbs) with L5 pedicle screw fixation. The L5–S1 interspace mobility was assessed on the basis of L5 tilt; patients with more than 15° of L5 tilt were instrumented with a standard unit rod construct. McCall and Hayes [26] found in follow-up that the patients that were instrumented to L5 with the U-rod had similar results to those fused with the standard unit rod construct.
12.6.2 Early-Onset Scoliosis in CP
A severe curve in pre-pubertal growth period presents a management dilemma. The options include continued observation, surgical intervention with growth-friendly spine implants to control the curves, or premature spinal correction and fusion.
The experience of growing rods in 27 children with CP at the mean age of 7.6 years showed 47 % correction of the Cobb angle from a mean of 85° [27]. The multicenter study highlighted complications in 19 of the 27 patients. Eight patients experienced deep wound infection (8/27, 30 %). Other complications include rod-related complications (11 occurrences) and anchor-related complications (six occurrences) in this group of vulnerable patients. Eight patients in the cohort had no complications and had four rod lengthenings at an interval of 11 months [27]. A similar complication rate was noted with the “Eiffel Tower” VEPTR construct [28].
Early spinal fusion in a cohort of 33 patients with a mean age of 8.3 years and mean curve of 85° with a minimum follow-up of 5 years was reviewed. Patients with early-onset scoliosis in this group of neurologically severely involved patients (31 patients GMFCS V) had a 28 % mortality rate, and six patients died between 1 and 5 years and 2 died between 10 and 15 years after surgery. Deep infection was reported in three patients (3/33, 9 %) [29].
Clearly, the ideal management plan is yet to be determined. An optimal surgery should be a growth-friendly spinal implant without the need for subject patients to undergo repeated surgeries whether for lengthening purposes or implant-related problem. A magnetic-driven growing rod has recently been FDA approved and can lengthen without surgery. Its role in early-onset spinal deformity in children with CP is yet to be defined.
12.6.3 Sagittal Plane Deformities
Sagittal plane deformities such as hyperkyphosis or lordosis may develop in patients with neuromuscular disorders, either with or without scoliosis. Flexible, postural deformities may be addressed in younger patients with tight hamstrings by lengthening the posterior thigh musculature and addressing the associated posterior pelvic tilt and pelvic retroversion in these patients or by appropriate modifications to the wheelchair or shoulder harness, but in older children, these adaptations do not work as well.
The spinal column lengthens with lumbar hyperlordosis correction and shortens with thoracic hyperkyphosis correction. Exclusion of a tethered cord is important prior to embarking on the surgical correction of lumbar hyperlordosis. Patients who have undergone a previous dorsal rhizotomy for spasticity can be at particular risk for developing a pathological hyperlordosis and associated spondylolisthesis. This has implications during posterior surgical exposure. The authors have experience with postoperative radiculitis after correction of hyperlordosis and relative lengthening of the lumbar spine with presumable nerve root tension.
Lumbar hyperlordosis and its associated pelvic anteversion and obliquity alter the trajectory of the pelvic fixation significantly and can be a risk factor for pelvic fixation-related complications [6]. Medial breach of the ilium resulting in bowel perforation by the limb of a unit rod has been described. A modular screw-based system is recommended [30] to decrease morbidity with pelvic screw placement, allow customization, and afford deformity correction.
12.6.4 Pelvic and Infrapelvic Coronal Plane Deformities
Compensatory scoliosis arises from coronal plane deformities of the pelvic and infrapelvic origin. Asymmetrical forces of the gluteus medius and hip adductors coupled with infrapelvic pathology such has hip subluxation and dislocation contribute to pelvic obliquity. In young patients, soft tissue procedures such as adductor and iliopsoas release could be attempted to achieve coverage of the femoral head and level the pelvis. With growth, the deformities can become stiff and need to be addressed by osteotomy of the proximal femur and pelvis. In such cases, spine surgery to restore spinal balance and pelvic obliquity is performed prior to the osteotomy of the pelvis for femoral head coverage.
12.6.5 Intraoperative Neuromonitoring
Spinal cord monitoring with intraoperative transcranial motor-evoked potentials and somatosensory-evoked potentials is controversial in this population [31] since meaningful monitoring is difficult. Up to 30 % of the patients with severe CP may have weak or absent signals at baseline, particularly transcranial motor-evoked potentials in the most severely affected children [32, 33].
Intraoperative neuromonitoring changes present a significant management dilemma. The Stagnara wake-up test is usually not possible. In the subgroup that responds to intraoperative optimization of physiological parameters and surgical correction, it could potentially advert neurogenic bladder (requiring urinary catherization) and maintain protective sensation even in the most neurologically involved patients. In the subgroup that has lost signals despite optimization, staging the procedure in this medically challenging group versus in situ correction is debatable. Problematically, the patients may not have reliable signals during the staged procedure. Involvement of the family in the potential decision-making is helpful to determine the course of action.
12.6.6 Anterior Release
Anterior release at the apical levels is indicated for stiff curves or curves greater than 90° not reducible with a pull or fulcrum bend film to gain flexibility and allow correction. Anterior release at the lumbosacral region includes psoas muscle recess at its origin, annulus release, and complete anterior discectomy, which helps with correction of pelvic obliquity and pelvic tilt [34]. With anterior surgery, complications and morbidity increase. Keeler et al. reported significantly higher infection, pulmonary and cardiovascular (coagulopathy or hypotension) complications when anterior release was employed [35]. Thoracoscopic anterior release is possible from the intervertebral disc of T4/5 to T11/12 and could reduce the operative time and morbidity associated with open thoracotomy.
It is unclear whether to stage the anterior and posterior procedures separately (1–2 weeks apart) or to do both the procedures on the same day. Evidence exists to support both strategies, and it is our practice to stage surgeries for patients with severe involvement and multiple medical comorbidites [36]. For relatively healthy patients, we usually perform both stages on the same day, provided that the time under anesthesia or blood loss is not too substantial after the anterior release. Anterior fusion for the so-called crankshaft phenomenon is not necessary, even for young patients, when rigid, segmental instrumentation such as a unit rod or pedicle screws are used posteriorly [37–39].
12.6.7 Intraoperative Halofemoral Traction
Intraoperative halofemoral traction is useful particularly in patients with kyphoscoliosis or significant pelvic obliquity [35, 40, 41]. Its use, however, is less optimal in patients with lumbar hyperlordosis where the traction on both legs may aggravate the lordosis. Anecdotal experience suggests that unilateral traction prior to corrective maneuvers is useful in leveling the pelvis.
12.6.8 Intrathecal Baclofen Pump
Intrathecal baclofen pump therapy is increasingly being employed to control muscle spasticity while maintaining muscle function. For patients with intrathecal baclofen therapy, great care is taken to ensure adequate padding at the site of the pump during prone positioning. The concurrent insertion of the pump with the spinal deformity surgery does not increase the rate of infection, and simultaneous procedures are not substantially difficult [42]. No significant cerebral spinal fluid (CSF) leakage is expected during insertion of the intrathecal component of the tubing. The pump including the connecting tubing at the intrathecal sac can be safely inserted or exchanged even post-spinal fusion below the conus medullaris.
12.7 Surgical Evolution and Outcome
Spinal instrumentation and fusion are indicated for collapsing deformities and painful sitting when no other alternatives exist [43]. Historically, fusions with Harrington instrumentation had an unacceptably high rate of pseudarthrosis in 18–27 % of cases [5, 17, 44, 45]. The advent of segmental instrumentation with Luque rod and sublaminar wiring yielded improved results over the Harrington system [19, 21, 46–48] and obviated the need for prolonged postoperative casting. Comstock et al. [17] found a mean correction of 51 % in a posterior-only instrumentation cohort 57 % and in an anterior–posterior cohort.
Multiple authors have noted progression of pelvic obliquity if the fusion was not extended to the pelvis [17, 19, 23]. The Galveston technique to extend the fusion across the pelvis by placing each Luque rod between the pelvic tables [49] demonstrated acceptable fusion rates across the L5–S1 segment and provided good control of pelvic obliquity. It was associated with a high incidence of loosening secondary to micromotion at the ilium at the sacroiliac joints, which was described radiographically as the “windshield-wiper” effect. While the impaction of two Luque rods into the pelvis with associated segmental fusion via sublaminar wires provides a strong construct in the sagittal plane, there exists a moment arm of rotation about the two rods allowing for rod translation with respect to one another, loss of torsional control, and subsequent progression of pelvic obliquity, pseudarthrosis, and implant failure [50]. The use of Luque rods smaller than one-fourth-inch diameter may increase the incidence of implant failure [21, 23, 51], but the intraoperative bending of one-fourth-inch diameter steel rods to the optimal geometry for pelvic implantation presents a technical challenge. Lonstein et al. found in a cohort of 93 patients a 50 % correction of the major scoliotic curve with a mean preoperative scoliosis of 72° and 40 % correction of pelvic obliquity at a mean follow-up of 3.8 years using a dual Luque-Galverston instrumentation technique [52]. With a similar construct, Sanders et al. [23] found that postoperative residual curve greater than 35°, preoperative curves greater than 60°, crankshaft deformity, and not fusing to the pelvis were the factors associated with postoperative curve progression. It is clear that rigid fixation is essential for surgical success.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








