Chapter 51C Vascular Diseases of the Nervous System
Intracranial Aneurysms and Subarachnoid Hemorrhage
An intracranial aneurysm is defined as an abnormally circumscribed dilatation of an artery which grows and ruptures over time. The term aneurysm originally comes from the Greek aneurysma—ana- meaning “across” and eurys meaning “broad.” Subarachnoid hemorrhage (SAH), mostly from rupturing of intracranial aneurysms, accounts for 5% to 10% of all stroke cases and is related to greater fatality than the other forms of strokes. Case fatality of aneurysmal SAH ranges between about 30% and 70%, with an average of approximately 50%. In more than half of SAH survivors, the level of disability is major; 60% of those patients never achieve the quality of life they enjoyed before the aneurysm ruptured (Kelly et al., 2001; Ropper and Zervas, 1984). In the United States, the estimated lifetime cost (including hospitalization, treatment, morbidity, and mortality) for patients hospitalized with unruptured intracranial aneurysms is $522 million, and $1.75 billion for patients with aneurysmal SAH (Wiebers et al., 1992). Owing to recent advancements in minimally invasive neuroimaging technology, such as computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), intracranial aneurysms are more frequently detected prior to rupture. Considering that intracranial aneurysm is a fairly common disease and the relatively young, productive age at which a disabling or fatal aneurysmal SAH could occur, in-depth understanding of intracranial aneurysms and their complications is very important for a modern comprehensive management of aneurysm patients.
Epidemiology
The true incidence of intracranial aneurysms remains unknown, but the reported incidence varies from as low as 0.2% to 10% of the general population, depending on whether the source of the data is an imaging study or an autopsy study. Some autopsy studies estimate that approximately 5% of the population may harbor undiscovered intracranial aneurysms (Jakubowski and Kendall, 1978; Juvela et al., 1993; Wiebers et al., 1992). Fortunately, most intracranial aneurysms do not rupture during the lifetime of a patient. Clinically, the frequency of ruptured aneurysms has been estimated at a median value of 11 per 100,000 people per year. In 1981, the Cooperative Study estimated the incidence of aneurysmal SAH in the United States at 26,000 cases per year (Sahs et al., 1981).
The prevalence of aneurysmal multiplicity ranges from 10% to 30% in large clinical series (Inagawa, 1990; Juvela, 2000; Qureshi et al., 1998). Multiple aneurysms can be mirror aneurysms or located asymmetrically on different locations in the circle of Willis. There is a strong female predominance in aneurysm multiplicity. Some studies have shown that multiple aneurysms are associated with vasculopathies such as fibromuscular dysplasia.
There is a rising occurrence of intracranial aneurysms associated with increasing age and female gender (Nakagawa and Hashi, 1994). In the United States, the peak age for aneurysm rupture is between 50 and 60 years of age (Sahs et al., 1981). Overall, the female-to-male ratio is approximately 2 : 1 in adults. Pediatric aneurysms are rare and account for about 1% to 3% of aneurysmal SAH (Wojtacha et al., 2001). In contrast to the definitive female predominance in adults, female-to-male ratio is reversed in the pediatric population. Large and giant intracranial aneurysms are more commonly reported in pediatric aneurysm series, particularly in infants (Meyer et al., 1989). These differences in clinical manifestations strongly suggest that the underlying etiology of intracranial aneurysm formation is different between pediatric and adult populations.
Pathophysiology
Saccular Aneurysm
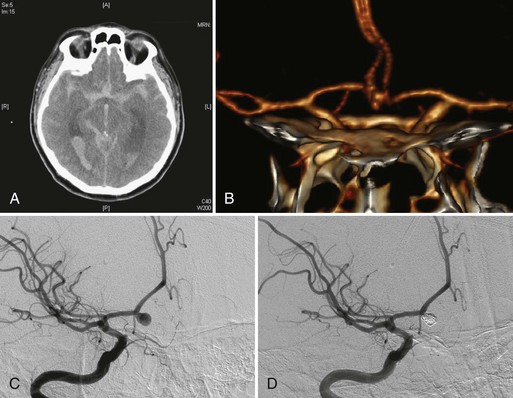
Intracranial aneurysms are classified according to their morphology, size, pathogenesis, and anatomical location (Box 51C.1).The most common intracranial aneurysm morphology is the saccular aneurysm, which is a rounded berry-like outpouching arising from first- and second-order branches in the circle of Willis (Fig. 51C.1). Saccular aneurysms account for approximately 90% of intracranial aneurysms and are responsible for most of the morbidity and mortality from SAH (Yong-Zhong and van Alphen, 1990).
Intracranial arteries are structurally unique in that they lack external elastic lamina. The disappearance of the external elastic lamina occurs in the horizontal segment of the cavernous portion of internal carotid arteries (Masuoka et al., 2010). Significant reduction or disappearance of elastic fibers in the tunica media and external elastic lamina also occurs in the vertebral arteries as they enter the skull (Wilkinson, 1972). Thus, the internal elastic lamina provides a very important structural support for the cerebral vasculature. Alterations in the internal elastic lamina or tunica media of intracranial arteries structurally weaken the arterial walls and make them less resistant to constant hemodynamic forces such as dynamic pressure, blood pressure, and shear stress. Although the etiology of intracranial saccular aneurysm formation involves multiple factors, the most significant pathogenetic factor is considered to be the degeneration of tunica media and internal elastic lamina at the branching sites of intracranial arteries in regions of chronic hemodynamic stress. In the pediatric population, the abnormalities of tunica media and internal elastic lamina may be congenital rather than the degenerative acquired changes in adults.
Fusiform Aneurysm
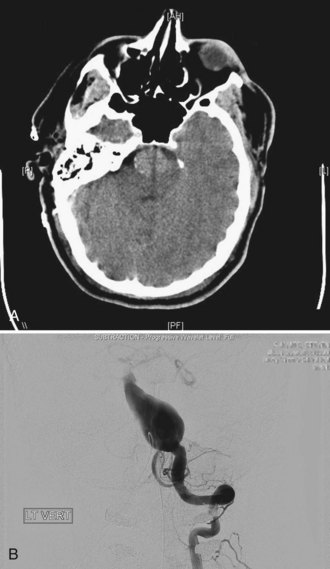
Two other morphological types of intracranial aneurysms are fusiform and dissecting aneurysms (see Box 51C.1), although the definitions of both aneurysm types may overlap. A fusiform aneurysm is defined as a circumferential dilatation in a segment of an intracranial artery. Unlike a saccular aneurysm that has a single orifice (neck) through which blood flow goes in and exits the aneurysm cavity, a fusiform aneurysm does not have a defined orifice (neck). The inflow and outflow of fusiform aneurysms are longitudinally separate, which often makes surgical or interventional treatment of these aneurysms challenging. Fusiform aneurysms are generally associated with atherosclerosis, which causes extensive damage to the media of an artery and results in arterial stretching to all sides or elongation. Although infrequent, other diseases are known to cause vessel damage resulting in fusiform aneurysms. If an arterial fusiform dilatation is accompanied by a marked elongation, it is call a dolichoectasia (dolichos, long; ectasia, distended). Dolichoectatic fusiform aneurysms are often seen in the posterior circulation and can reach several centimeters in diameter (Fig. 51C.2). Patients with dolichoectasia aneurysms characteristically present with mass effect on cranial nerves or brainstem compression, but they are not commonly associated with subarachnoid hemorrhage (Lou and Caplan, 2010).
Dissecting Aneurysm
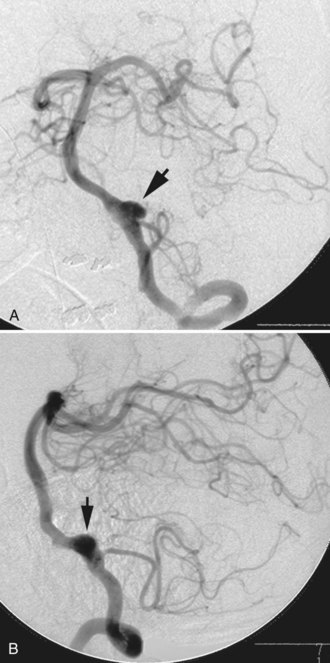
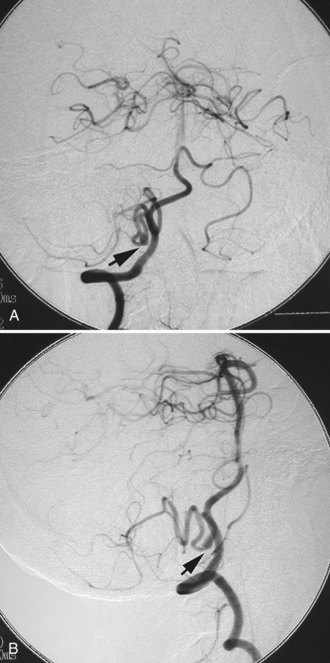
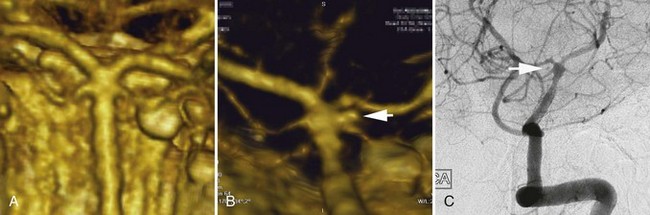
A dissecting aneurysm is formed as a result of splitting or dissection of an arterial wall by blood flow entering through a tear. This may cause a ballooning out on one side of the artery, with arterial narrowing (a classic radiographic appearance of pearl-and-string sign), an outpouching mimicking a saccular aneurysm (pseudoaneurysm or false saccular aneurysm), or a fusiform segmental dilatation. It could obstruct blood flow through the dissecting segment of an artery. Consequently, the angiographic and physical appearance of an intracranial dissecting aneurysm or arterial dissection varies widely depending on the arterial layer in which the dissection occurs and the extent of the dissection (Fig. 51C.3). Intracranial dissecting aneurysms often present with hemorrhage. In patients with severe SAH but no clear angiographic evidence of the source of the hemorrhage, very careful review of the angiograms should be conducted to search for a small dissecting aneurysm (Figs. 51C.4 and 51C.5).
Intracranial dissections can occur spontaneously, but they are commonly associated with trauma or an underlying vasculopathy such as fibromuscular dysplasia. Although controversial, it is believed that trivial trauma such as head turning, chiropractic manipulation, sneezing, or any sports activity may cause intracranial dissection or resultant dissecting aneurysm, with or without underlying vasculopathies (Smith et al., 2003). Unlike saccular aneurysms in the adult population, there is a male predominance in intracranial dissecting aneurysm (Yamaura et al., 1990).
Associated Conditions
Various hereditary and acquired risk factors are known to be associated with the formation of intracranial aneurysms. Some of the conditions listed in Box 51C.2 are controversial and still being studied. Nevertheless, any congenital or acquired condition that produces vascular wall weakening and/or increased hemodynamic stress could result in the formation of intracranial aneurysms. Approximately 5% of intracranial aneurysms are associated with heritable connective tissue disorders, the most important being Ehlers-Danlos syndrome vascular type (type IV), neurofibromatosis type 1, and autosomal dominant polycystic kidney disease (Schievink, 1997). The association of intracranial aneurysms and autosomal dominant polycystic kidney disease has been well studied. The prevalence of intracranial aneurysms in patients with polycystic kidney disease is approximately 8%, which is higher than in the normal population (Rinkel et al., 1998). Positive family history of aneurysm in a patient with polycystic kidney disease increases the risk (Pirson et al., 2002). The vascular type of Ehlers-Danlos syndrome, formally called type IV, has a defect of type-III collagen synthesis, which is a major component of distensible tissues such as vessels. A higher incidence of intracranial aneurysms, particularly in the cavernous segment of internal carotid artery, has been reported. The indication of arterial or venous puncture for catheter angiography in patients with Ehlers-Danlos syndrome should be carefully discussed because of the vascular fragility.
Box 51C.2 Risk Factors and Associated Conditions for Intracranial Aneurysms
Familial intracranial aneurysms are defined as those identified in two or more first-degree relatives. Studies have shown that the risk for harboring intracranial aneurysms in such families is higher than in the general population. This is not just due to genetic factors but also could be due to common environmental exposures. The reported incidence of familial intracranial aneurysms varies but is approximately 8% in persons with two or more relatives who have had a subarachnoid hemorrhage or an aneurysm (Ronkainen et al., 1997).
The prevalence of intracranial aneurysms increases with age in both genders. This is not surprising, since chronic hemodynamic stimuli and degenerative changes in intracranial arteries play a role in aneurysm formation. Many studies have shown that current cigarette smoking is a risk factor for intracranial aneurysm formation (Inagawa, 2010), and it is suggested that cigarette smoking may increase the risk of aneurysm rupture. Cocaine use is a known risk factor for intracranial aneurysm formation. This association is likely due to the transient increase of blood flow and blood pressure. Among cocaine users, intracranial aneurysms are found in a younger population. The role of hypertension in the formation of intracranial aneurysms has been controversial in the literature. However, coarctation of the aorta, pheochromocytoma, and cocaine use have been associated with intracranial aneurysms, most likely because of the elevated blood pressures that occur in these conditions. Considering hypertension is associated with degenerative changes such as intracranial atherosclerosis, it could play a role in a subset of intracranial aneurysms.
Natural History of Intracranial Aneurysms
Surgical or endovascular treatments of unruptured incidental intracranial aneurysms remain controversial. Thorough understanding of the natural history of incidental intracranial aneurysms as well as the risks associated with their repair is mandatory for the appropriate management of aneurysm patients. The International Study of Unruptured Intracranial Aneurysms (ISUIA) group investigated the outcome of unruptured intracranial aneurysms in patients who underwent surgical treatment or no treatment (conservative observation). Two ISUIA studies, the largest series to date, were published in 1998 and in 2003, and both reported a lower incidence of aneurysm rupture and a higher incidence of procedural morbidity and mortality than most practicing clinicians expected (ISUIA Investigators, 1998, 2003). The first study published in the New England Journal of Medicine in 1998 was highly controversial for many reasons, but mainly because of its selection bias and the inclusion of many cavernous-carotid aneurysms.
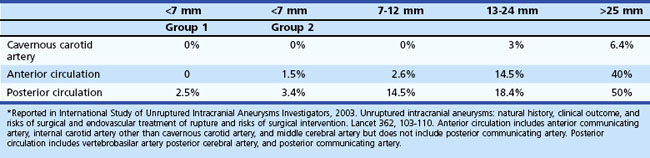
The second study published in Lancet in 2003, which was less controversial to practicing physicians, prospectively analyzed 4060 patients for treatment outcomes versus conservative observation. The untreated cohort consisted of 1692 patients with 2686 aneurysms, 1077 with no prior history of SAH (classified as group 1), and 615 with a prior history of SAH from another aneurysm (classified as group 2), with a mean follow-up period of 4.1 years. Among this cohort, 51 patients (3%) had a confirmed SAH (Table 51C.1). The study reported that larger aneurysm size was associated with higher risk of rupture in group 1 aneurysms but not in group 2, although there were only a few large aneurysms in group 2. According to their data, the rupture rate of group 1 aneurysms was extremely low (<0.1% per year). The ISUIA also found that posterior circulation aneurysms, including posterior communicating artery aneurysms, carried higher rupture rates than anterior circulation aneurysms.
It should be emphasized that the ISUIA results do not apply to all situations, not only because of their non-randomized nature and selection bias but also for other reasons. For instance, fusiform aneurysms, saccular traumatic aneurysm, and mycotic aneurysms were excluded from the ISUIA study, so the true natural history of such aneurysms remains unknown. Furthermore, saccular aneurysms with an irregular contour or an obvious bleb formation may have been included in the ISUIA very infrequently, since the enrolling physicians felt it unsafe to conservatively follow such irregular-shaped saccular aneurysms. Moreover, among small saccular aneurysms (<7 mm) that could have been included in the ISUIA study, the rupture risk of ISUIA no longer applied once unruptured aneurysms increased in size during the follow-up (Juvela et al., 2001; Miyazawa et al., 2006). The growth of an aneurysm may warrant immediate intervention regardless of its size.
The result of the second ISUIA in 2003 may lead to the conclusion that a patient with a group 1 aneurysm less than 7 mm in the largest diameter does not benefit from any treatments, including endovascular coiling. On the other hand, most ruptured aneurysms in many reports are less than 7 mm in the greatest diameter, and there have been no clear scientific explanations for this discrepancy. Also, the ISUIA results are not in direct accordance with previous natural history studies (Juvela et al., 1993; Tsutsumi et al., 2000). The cumulative risk of aneurysm rupture reported in most other clinical studies ranges between approximately 10% and 20% at 10 years, and the risk becomes higher for large (>10 mm) aneurysms.