Chapter 173 Vertebroplasty and Kyphoplasty
Indications and Techniques
Before the introduction of vertebroplasty and kyphoplasty, treatment of compression fractures consisted of bed rest, pain control (with nonsteroidal anti-inflammatory medications, calcitonin, and narcotics), and back bracing.1 This regimen is often effective, and most people eventually find relief from their pain as the fracture heals. However, a substantial number fail to heal within 3 to 6 weeks. These people often suffer persistent back pain and a risk of gradually worsening kyphotic deformity. Even in those whose compression fractures heal over time, the recovery period is time consuming, requiring at least several weeks. The experience is often profoundly unpleasant and can be marked by periods of insufficient pain control, delirium, and constipation. Such patients are sometimes bedridden, with the attendant risks of deep venous thrombosis, pulmonary embolism, and pneumonia. The prolonged immobility accelerates bone resorption and predisposes the patient to additional injury. Finally, the long-term effects of even a healed fracture may be substantial: kyphotic deformity, decreased lung capacity, and altered forces on intervertebral discs and facet joints.
The vertebroplasty procedure was introduced in France in 1984 by a group that used the technique to treat symptomatic vertebral hemangiomas.2 They found that the “internal casting” provided by polymethyl methacrylate (PMMA) injected into the pathologic vertebral body provided substantial pain relief. This success led to expansion of the procedure to the treatment of pain from myeloma and metastatic neoplasms of the vertebrae3; these indications remain the most common ones for the use of vertebroplasty in Europe. In 1993, the technique was introduced in the United States,4 where its chief use has been to treat the pain from osteoporotic compression fractures. Kyphoplasty was introduced in 1999 as the proprietary technology of Kyphon Inc. Kyphoplasty differs from vertebroplasty by adding the insertion and inflation of a balloon before cement delivery. It has the added goal of restoring vertebral body height and spine alignment.5 Both procedures are now practiced throughout the world, with the number of procedures and practitioners (including radiologists, orthopedic surgeons, neurosurgeons, and anesthesiologists) growing rapidly.
Contraindications
In addition, specific fracture features do not represent absolute contraindications but substantially increase the risk or technical difficulty of the procedure. Disruption of the posterior cortex increases the risk of posterior cement leakage and therefore the risk of spinal cord or nerve root compression; this feature is rare in osteoporotic compression fractures but frequent in burst fractures and metastatic neoplasm. Substantial canal narrowing (without clinical evidence of myelopathy or radiculopathy) increases the risk that even a small amount of cement leakage will produce neurologic compromise. Marked loss of vertebral body height makes the procedure more difficult since there may be little space for needle placement. Poor visualization of osseous structures on fluoroscopy increases the risk of improper needle placement and symptomatic cement leakage but can be overcome with the use of computed tomography (CT). Treatment of patients whose fractures have these features should only be performed by the most experienced practitioners.
Preprocedure Evaluation
History
The classic symptoms from an acute vertebral compression fracture include deep pain with sudden onset, midline location, and exacerbation by motion and standing. These fractures may occur with little or no trauma. The pain often diminishes little in the first week and then fades gradually over the next several weeks to 3 months.1 Lateral radiation may be present, but persistent radiation of the pain in a radiculopathic pattern is rare. When there is substantial kyphosis, patients may also suffer from difficulty breathing, anterior chest wall pain, and gastrointestinal discomfort.
Physical Examination
The practitioner should perform a directed physical examination. This examination includes inspection of the back, palpation for focal areas of tenderness, and correlation of the site of pain with anatomic landmarks. In difficult cases, examination of the sites of pain and tenderness can be performed with fluoroscopic assistance to localize the pain to specific anatomic structures. Although point tenderness at the spinous process of the fractured vertebra is the typical finding in unhealed compression fractures, the absence of typical focal tenderness does not preclude the presence of such a fracture. Imaging is a more reliable test than this portion of the physical examination.6 Assessment of lower extremity neurologic function is especially important in patients with symptoms suggestive of myelopathy, radiculopathy, or spinal stenosis. Evaluation of the heart and lungs is necessary for the safe use of sedation for the procedure.
Imaging
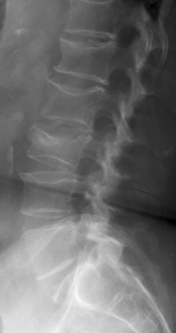
Imaging of the spine is undertaken in all cases to confirm the clinical diagnosis and to plan the procedure. Radiographs are the imaging study of choice for the initial imaging evaluation (Fig. 173-1). Anteroposterior (AP) and lateral views should be obtained. This study allows the practitioner to confirm the presence of fracture, determine the location of fractures, assess the degree of height loss and kyphosis, and identify anatomic variants. Whenever possible, comparison to prior studies is valuable. A single radiograph almost never allows the practitioner to distinguish a new or unhealed fracture from a chronic, healed fracture. With prior radiographs (“old gold”) for comparison, new compression fractures can be identified. If serial radiographs taken several weeks apart show a new compression fracture in a patient with classic history and symptoms, no further imaging may be necessary. However, since back pain is a difficult diagnostic problem, most patients benefit from advanced imaging.
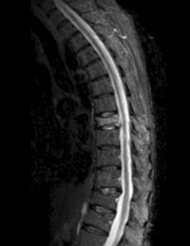
Magnetic resonance imaging (MRI) is the test of choice for this additional evaluation. The main goals of MRI are to (1) distinguish new or unhealed fractures from healed fractures, (2) identify other causes of pain, and (3) evaluate the risk of symptomatic cement leakage during the procedure. For these purposes, the most useful sequence is a T2-weighted sequence with fat suppression. We prefer using inversion recovery (i.e., a short tau inversion time recovery, or STIR, sequence) to achieve the fat suppression, since it is less prone to artifact than frequency-selective fat saturation techniques. On these MRI sequences, recent or unhealed fractures show a hyperintense signal within the bone marrow (Fig. 173-2). This signal may be present in poorly marginated areas filling much of the vertebral body or as discrete curvilinear fracture clefts. In contrast, healed fractures have a marrow signal similar to that of unfractured vertebra. These sequences also provide an adequate evaluation of the spinal canal and show most alternative causes of pain that might mimic fracture. Additional imaging with a sagittal T1-weighted sequence and axial T1- and T2-weighted images may be helpful. In most patients with a typical clinical history and physical examination for osteoporotic compression fracture, the STIR sequence alone is adequate, and the practitioner can reserve the full set of MRI sequences for patients whose pain is atypical or who have STIR abnormalities that require clarification. We have found value in obtaining an additional MRI shortly before the procedure when either (1) there has been worsening of the patient’s pain since the initial evaluation or (2) the imaging study used for the initial evaluation is more than 3 months old.7
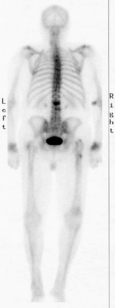
In patients who cannot tolerate MRI (e.g., those with a pacemaker), bone scan is the test of choice. Bone scan does not provide any evaluation of the soft tissues or spinal canal, but it does allow the differentiation of healed and recent fractures. The recent fractures take up the injected technetium 99m–medronate tracer in much higher concentrations (Fig. 173-3). The relative sensitivity and specificity of bone scan and MRI for this purpose have not been established.
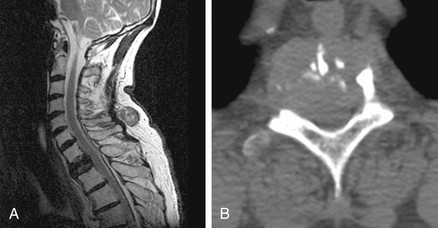
CT is not necessary for most osteoporotic compression fractures but is useful for preprocedure evaluation of burst fractures and metastases. In these circumstances, there may be substantial loss of integrity of the posterior vertebral body cortex (Fig. 173-4). This loss increases the risk of the procedure, specifically the risk of posterior leakage of cement or posterior displacement of bone or tumor.8 CT provides the best means for assessing this cortex. CT is also the test of choice for postprocedure evaluation of unexpected symptoms (see the later section on risks).
Procedure: Vertebroplasty
Sedation
Analgesia is necessary to perform vertebroplasty and kyphoplasty safely and with minimal discomfort. This process begins with the informed consent. Fully informing the patient of what to expect in the operating room or procedure suite facilitates patient comfort, cooperation, and satisfaction. Satisfactory analgesia usually can then be achieved with local anesthetics and moderate sedation, but in some cases general endotracheal anesthesia is needed. Vertebroplasty has generally been performed with moderate sedation, while kyphoplasty has more commonly been performed with general anesthesia. At our institution, both are usually performed using moderate sedation with intravenous midazolam and fentanyl. Continuous patient monitoring is performed with electrocardiography, blood pressure measurement, and pulse oximetry. The drug delivery and monitoring are performed by certified nursing personnel. In patients with substantial preexisting respiratory or cardiac disease, an anesthesiologist is asked to evaluate the patient and determine whether monitored anesthesia care is warranted.
Patient Positioning
The ideal patient position for thoracic and lumbar procedures is prone. This position allows the practitioner to place the needle from either side and simplifies positioning of the biplane fluoroscopy unit or C-arm. Furthermore, this position and proper cushion support maximize extension of the fractured segments, promoting kyphosis reduction.9 Although the patient can be rotated slightly, substantial patient angulation can make this a more difficult procedure. A special table and cushions may be used to support the head and body, but good results can be achieved with a flat fluoroscopy table and careful placement of cushions. The patient’s arms should be placed sufficiently toward the head to keep them out of the path of the fluoroscope. Patients can usually be placed in the prone position despite their painful compression fracture. If necessary, moderate sedation can be initiated before the patient is moved. Care must be taken when transferring aged or osteoporotic patients, since transfer alone may cause new fractures.
Needle Placement
For either approach, there are two image guidance strategies. The “end-on” approach uses fluoroscope angulation to place the barrel of the pedicle cortex over the target site in the vertebra body. This view simplifies trajectory planning and is the simplest way of preventing the needle from violating the medial cortex of the pedicle. Alternatively, the AP approach may be used. This view may be easier to achieve with some fluoroscopy systems and in some cases provides superior delineation of the pedicle margins. Once an approach has been selected, the skin is anesthetized with a small amount of subcutaneous lidocaine or bupivacaine. Following the planned needle trajectory, the periosteum is anesthetized in a similar fashion. The planned trajectory can be assessed and adjusted from fluoroscopic views of this smaller needle at the bone surface. A small nick is made in the skin, and an 11- or 13-gauge needle is placed.
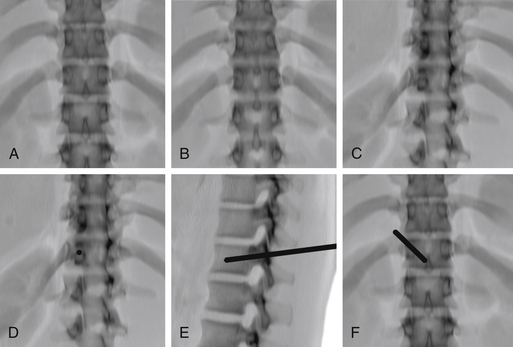
End-on Technique
For the end-on technique, the operator rotates the fluoroscopy unit into an ipsilateral oblique view that places the fluoroscopy beam and needle tract perfectly parallel to each other (Fig. 173-5). The image intensifier is first rotated to a true AP position, aligning the spinous process midway between the pedicles. The craniocaudal angulation is changed to bring the pedicles to the midportion of the vertebral body. The image intensifier is then rotated to bring the ipsilateral pedicle so that its medial cortex is at the middle third of the vertebral body. This rotation can only be continued as long as the medial cortex of the pedicle remains clearly visible. The needle is placed so that it is “end on” to the image intensifier and appears as a small circle or ellipse. Once the needle has been advanced to the bone surface, small corrections in the craniocaudal angulation can be made using a true lateral view (care is needed to angle this view so that the pedicles project over each other). The needle is then advanced through the pedicle, maintaining the end-on appearance of the needle. Once the needle has traversed the pedicle, it is advanced using the lateral view to the junction of the anterior and middle thirds of the vertebral body.
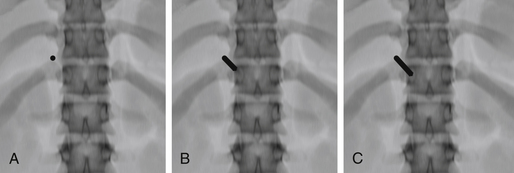
AP Technique
For the AP technique, the craniocaudal angulation is adjusted to bring the vertebral body end plates perpendicular to the image (Fig. 173-6). The skin entry site is placed about 1 cm superior and lateral to the center of the pedicle but must be adjusted for the thickness of the posterior paraspinal soft tissues. The needle is advanced anteriorly, medially, and caudally. By the time it reaches the bone surface, the tip should project over the upper outer cortex of the pedicle. Again, small corrections in the craniocaudal angulation can be made using a true lateral view. The needle is advanced so that its tip projects over the center of the pedicle on both AP and lateral views. The tip should project over the medial pedicle cortex as the needle traverses the posterior third of the vertebral body. The final position is again adjusted on the lateral view.
The image guidance for a parapedicular approach is quite similar. The needle is placed lateral and superior to the lateral cortex of the pedicle and enters the vertebral body at its junction with the pedicle. The position at which bone is encountered (i.e., at the junction of the pedicle and vertebral body) is more anterior on the lateral view.
The planning for the lateral position of the tip should be adjusted for the procedure to be performed and the shape of the vertebral body. Vertebroplasty for osteoporotic vertebral compression fractures was initially performed using two needles and a bipedicular approach. This technique allows reliable filling of both halves of the vertebral body, independent of the precise lateral position of the needle tip within its half of the vertebral body. However, some practitioners have adopted a unipedicular approach. This technique requires a single needle and decreases the risk and time associated with needle placement. For the unipedicular approach, a near-midline position of the needle tip provides more reliable filling of the vertebral body. In my experience, both techniques work well, and my colleagues and I base our approach on the number of levels to be treated and the configuration of the fractured vertebra. For example, in a severely collapsed vertebra, there is often substantially more marrow space at the lateral aspects of the vertebral body. In this circumstance, a bipedicular approach with lateral positioning of the needle tip provides reliable bilateral filling with little risk of symptomatic cement leakage.10 With more lateral tip positions, it is important to remember that the vertebral body is round and that the anterior cortex of the vertebra along the needle trajectory is posterior to the radiopaque line of anterior cortex on the fluoroscopic image.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree