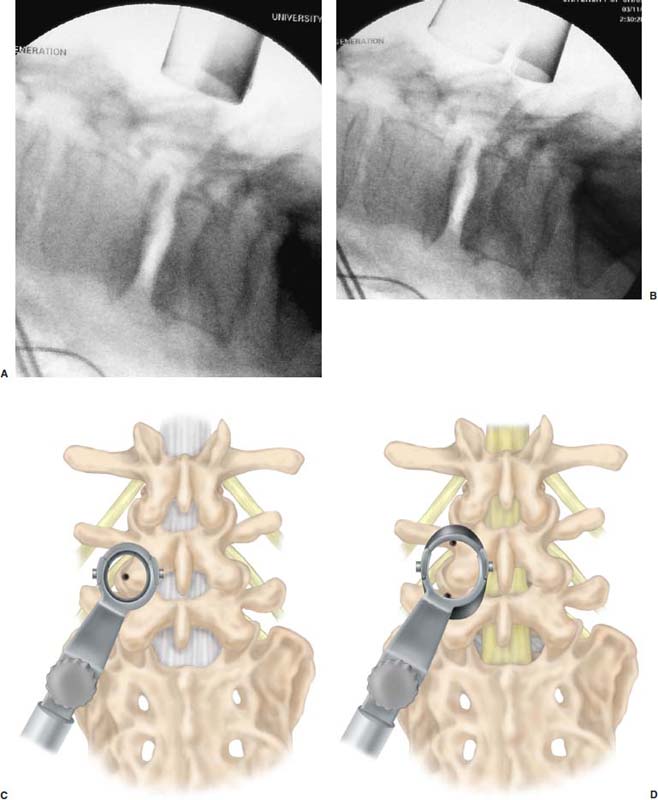
After 45 years of experience with lumbar spine surgery, Cloward,1,2 the first surgeon to successfully perform a posterior lumbar interbody fusion (PLIF), declared that PLIF should replace simple diskectomy, decompressive laminectomy, and chemonucleolysis as the definitive surgical procedure for the lumbar spine. Through a single dorsal approach, 360-degree stabilization of the lumbar spine is possible. This avoids a second anterior surgery and the morbidities associated with that approach. However, the procedure is technically challenging and requires significant retraction of the thecal sac and nerve root, which can cause injury and also limits surgical treatment to the lower lumbar spine. When performed properly, good outcomes have been attained in 80 to 85% of patients.2,3 Harms and Rolinger4 reported a modification of the PLIF procedure where a unilateral facetectomy was done and grafts were placed from a more lateral location.4 This transforaminal or TLIF technique was safer because it required little or no retraction of the thecal sac or nerve root while still accomplishing a circumferential fusion. Humphreys et al5 compared TLIF and PLIF procedures and found that PLIF was associated with significantly higher blood loss than TLIF; PLIF also resulted in multiple complications, whereas TLIF had no associated complications.5 When compared to anterior interbody fusion with posterior instrumentation, TLIF is associated with decreased blood loss, shorter operative times, and reduced cost.6,7 Significant disruption of the musculoligamentous complex occurs with open PLIF and TLIF procedures. Iatrogenic injury to soft tissue support structures of the spine have been negatively correlated with long-term fusion outcomes.8–12 In an effort to minimize damage to soft tissue but still achieve lumbar arthrodesis, a minimally invasive PLIF (MI-PLIF) technique was developed.13 As with the open PLIF procedure, MI-PLIF has significant challenges and risks. Although the senior author’s (RGF) initial experience with MI-PLIF was uncomplicated, it was felt that the procedure would be easier and safer if a transforaminal approach were used. The open TLIF procedure was modified and adapted to current minimally invasive methods. Herein we describe our technique for performing a minimally invasive TLIF (MI-TLIF). Indications Which fusion procedure (anterior, posterior, or both) is best to treat various lumbar spine diseases is the subject of considerable debate. PLIF has been successfully used to treat a broad range of lumbar pathologies including spondylolisthesis, segmental instability, failed back syndrome, recurrent disc herniation, massive bilateral disk herniation, disk herniation alone in a heavy laborer, and degenerative disk disease with mechanical back pain.14–16 The indications for TLIF are identical to those for PLIF. It is our practice to offer MI-TLIF fusion to our spondylolisthesis patients (grades I and II only) with demonstrable instability on dynamic radiographs. Patients with degenerative disk disease and mechanical back pain should have clear evidence of degenerative changes at the affected level or have reproducible symptoms on provocative testing, such as a diskogram. We generally do not recommend fusion for recurrent or massive disk herniations. Instead we recommend fragmentectomy of the disk using a microendoscopic technique. These patients may ultimately require a fusion, but by employing minimally invasive techniques, this may be postponed, perhaps indefinitely. Surgical Equipment/OR Setup In order to safely perform a minimally invasive TLIF procedure, several pieces of equipment are essential. C-arm fluoroscopy is a must. The use of image guidance is optional. At this time, it is not our practice to use image guidance for this procedure. An expandable tubular retractor (Ex-Tube, Medtronic Sofamor Danek, Memphis, TN) is very important for safe execution of a minimally invasive TLIF. The tube is inserted at a diameter of 26 mm and is expanded in situ to a final working diameter of 44 mm (Fig. 24–1). Use of an endoscope is optional; it is helpful for visualizing the nerve root when placing interbody grafts. We generally use loupe magnification with a headlight or the operating microscope during the procedure. The basic surgical set is essentially the same as a standard laminectomy/fusion except the instruments are slightly longer for working through the tubular retractor. It is important to have a high-speed drill (Midas Rex, Ft. Worth, TX) available as an aid for removing bone. The tools for readying the disk space for graft placement consist of distractors (7–14 mm), rotating cutters, endplate scrapers, and a chisel (Fig. 24–2A–D). Many options exist for interbody graft material. We have had good results when using either allograft bone or cages. Our current practice is to use a polyether ether ketone (PEEK) cage with bone morphogenetic protein-2 (BMP-2) (Medtronic Sofamor Danek). Marking the pedicles for placement of percutaneous screws is relatively easy and requires only an 11-gauge bone biopsy needle, a Kocher clamp, K-wires, a drill, and fluoroscopy. We use the Sextant instrumentation set (Medtronic Sofamor Danek) for placement of cannulated pedicle screws. The operating room is arranged such that the operating table is in the center of the room, anesthesia at the head, and fluoroscopy monitor at the foot (Fig. 24–3). The C-arm base is placed on the side opposite of the TLIF as is the video monitor. Equipment tables are kept behind the surgeon on the operative side and a Mayo stand is situated over the feet to pass instruments in active use. The patient is positioned prone on a Wilson frame, which is placed on a Jackson table (Fig. 24–3). It is helpful to use the Jackson table for ease of moving the C-arm during surgery. The arms are bent 90 degrees and placed alongside the patient’s head. The knees, axilla, elbows and wrists are padded to prevent nerve palsies. The legs are elevated with pillows to reduce stretch on the sciatic nerve. The face is placed in a padded mask that has a mirrored surface (Prone View, Dupaco) so the anesthetist can view the face and endotracheal tube throughout the procedure. Surgical Technique Following induction and intubation, a Foley catheter is placed and leads are inserted into appropriate lower extremity muscle groups for EMG monitoring. The anesthetist is instructed to avoid the use of paralytics, muscle relaxants, and nitrous oxide, which may interfere with EMG recordings. A single dose of antibiotics, either cephazolin or vancomycin, is administered. Sequential compression devices are placed on the legs and the patient is brought to the prone position on the operating table. The C-arm is brought into position for a lateral view of the affected level. An occlusive barrier is placed at the top of the gluteal cleft, and the skin is prepped and draped in standard fashion. Localization and Exposure The region of pathology is localized with the aid of fluoroscopy and a Steinmann pin. Once marked, a stab incision is made 3 cm from the midline, and the Steinmann pin is inserted until it rests on bone. Ideally, the pin should be on the facet complex of the affected level. If localization is satisfactory, the skin incision is extended to a final length of 2.5 to 3.0 cm with the position of the Steinmann pin being the center of the incision. Sequential dilators are passed over one another and fluoroscopy is used to confirm adequate insertion. The appropriate length working channel is introduced over all the dilators, brought into line with the disk space in a medial orientation, and secured to the operating table with a flexible arm clamp. The working channel is opened to its full capacity using the specially designed distractor, and when open, it should span the distance from pedicle to pedicle at the level of interest (Fig. 24–4A–D). Muscle and soft tissue are cleared from the lamina and facet with monopolar cautery (Fig. 24–5A). Next, the working channel is angled laterally, and the transverse processes are exposed. The tubular retractor is again turned medially to begin the laminotomy and facetectomy. Laminotomy/Facetectomy A straight curette is used to define the interlaminar space and a plane is developed between the ligamentum flavum and bone with an angled curette. Fluoroscopy is used sporadically throughout the procedure to assess position and evaluate the extent of decompression. Angled Kerrison ronguers are used to begin the laminotomy and facetectomy (Fig. 24–5B). We save all the bone for later use in the transverse process fusion. The decompression should extend from pedicle to pedicle in a rostral-caudal direction. Laterally, a near-total or total facetectomy is done to provide adequate space for graft placement (Fig. 24–5C). Next, the ligamentum flavum is removed (Fig. 24–5D). Epidural veins are coagulated with bipolar cautery and divided if necessary. The lateral edge of the dura, the nerve root, and the disk space should be clearly visualized (Fig. 24–6A–F). FIGURE 24–1 Sequential dilators (A) used to establish a working corridor to the spine. Ex-tubes in the closed (B) and open position (C). Interbody Fusion A 15-blade scalpel is used cut the anulus, and disk material is removed with pituitary rongeurs (Fig. 24–7A,B). A down-angled curette is helpful to ensure that subligamentous disk fragments and the contralateral disk are properly removed (Fig. 24-7C). Disk space dilators are then inserted to measure the interbody space for the appropriately sized graft. The disk space is sequentially dilated until disk space height is similar to adjacent levels. The maximum insertable dilator translates into the width of the interbody graft we use. Next, the rotating cutter is introduced parallel with the disk space and rotated to start preparing the vertebral body end plates (Fig. 24–8A). The end plates are scraped, and debris is removed with a pituitary rongeur (Fig. 24-8B). A chisel is used to remove osteophytes (Fig. 24–8C). Our experience has been that a chisel slightly smaller than the graft works best (e.g., a 10-mm chisel is used for a 12-mm graft). The operative site is copiously irrigated with antibiotic saline prior to graft placement. The end plates are now ready for graft placement (Fig. 24–9A–C). If allograft (Tangent, Medtronic Sofamor Danek) is used, we insert two grafts and orient them toward each other such that a circle in the center of the vertebral body is made on completion of graft placement (Fig. 24–10A–D). When placing the PEEK cage, we first lay a BMP-2 soaked sponge along the anterior annulus, place a BMP-2 pledget in the cage, and insert the cage obliquely such that it is centered in the disk space. Thrombin-soaked Gelfoam and a large cottonoid are placed over the interspace for hemostasis. Attention is next directed at the transverse processes. The tubular retractor is adjusted to visualize the transverse processes. They are decorticated with a high-speed drill and autograft bone saved from the laminectomy and facetectomy is packed between the processes. If the bone quantity seems inadequate, it is supplemented one-to-one with allograft cancellous bone chips. The working channel is collapsed and the cottonoid, Gelfoam, and working channel are removed. FIGURE 24–2 Instruments necessary for preparing the disk space for graft placement. (A) Disk space dilators (7–15 mm). (B) Rotating cutters. (C) Endplate scraper. (D) Chisel. FIGURE 24–3 Operating room setup for an MI-TLIF procedure. Instrumentation The next part of the procedure is insertion of the instrumentation. We use the “bull’s eye” technique for marking the pedicles. The C-arm is rotated 90 degrees for a true AP view parallel with the disk space. The bone biopsy needle is localized over the pedicle and passed through the soft tissue onto the pedicle. We try to target the center of the pedicle and then orient the needle so it is directly in line with the pedicle; the needle will appear as a single spot (“bull’s eye”) in this orientation (Fig. 24–11 A). The needle is tapped into the pedicle with a mallet and position is confirmed by fluoroscopy. Then, with the needle held firmly in the correct orientation, the stylet is removed and a K-wire is drilled ~1 cm into the pedicle. The bone needle is removed and fluoroscopy is used to confirm that the K-wire is in the center of the pedicle (Fig. 24–11 B). The process is repeated for the contralateral pedicle and then for both pedicles at the adjacent affected level. The C-arm is brought to the lateral position for advancement of the K-wires. We insert K-wires about two thirds the length of the vertebral body parallel with the end plate. The remainder of the procedure can be completed in less than 30 minutes. Soft tissue over the K-wires is dilated, the pedicles are tapped, and cannulated screws are inserted. Care is taken not to tap too far as this can dislodge the K-wire and necessitate beginning over. The Sextant device is attached to the screw extenders and pushed through the soft tissue to create a tract for the rod (Fig. 24–12A,B). The rod size is calculated by placing templates on the Sextant at this point. The tip of the Sextant is replaced with the rod, which is pushed through the soft tissue and both screw heads. The C-arm is brought to the AP orientation to confirm that the rod has passed through both screw heads before tightening. The screws are then compressed, tightened, and broken off with a torque wrench. The Sextant is disconnected from the rod and removed. The process is repeated on the opposite side. Wounds are irrigated with antibiotic saline and closed in layers with absorbable suture. The wound is dressed with three coats of 2-octyl cyanoacrylate (Dermabond; Ethicon). FIGURE 24–7 MI-TLIF preparation of the disk space. (A) The thecal sac and disk space are exposed following removal of the ligamentum flavum. (B) The diskectomy is begun. (C) The end plates are prepared. FIGURE 24–12 (A) Sextant apparatus attached to two screw extenders prior to passage of a rod. (B) The rod is passed subcutaneously. A number of complications are possible with this technique, but with care and anticipation, most can be avoided. It is possible for the Steinmann pin or the dilators to slip into the interlaminar space and cause dural perforation or nerve injury. This can be avoided by removing the Steinman pin after passage of the first dilator and ensuring that the dilators are docked on bone with passage of successive dilators. Dural tears and nerve root injury can occur with removal of bone and ligament. Good visualization and generous use of an angled curette to define and develop a plane are the best ways to avoid these complications. Intraoperative EMG monitoring provides immediate feedback of nerve irritation throughout the procedure, especially during graft placement. Frequent use of fluoroscopy is helpful to guide decompression and for accurate screw placement. Case Illustration A 60-year-old woman with chronic low back pain developed muscle spasms in her left buttock of 6 months’ duration. She had an episode of severe back and buttock pain that necessitated hospitalization for pain control. There was no history of prior back surgery or trauma. Physical examination revealed no abnormalities. Plain radiographs of her lumbosacral spine revealed grade I spondylolisthesis; MRI and CT scan demonstrated significant stenosis at L4–5 (Fig. 24–13A–E). She underwent a left L4–L5 MI-TLIF/Sextant with PEEK interbody graft, BMP-2, and left transverse process fusion. Good reduction of her spondylolisthesis was achieved, and the graft was in good position following surgery. Surgery was completed in 3.5 hours; blood loss was estimated at 100 cc. She was discharged from the hospital 3 days following surgery. Six weeks after surgery, she was off narcotic pain medications and had returned to work. Radiographs at this time showed good alignment of her spine and evidence of early fusion between the transverse processes (Fig. 24–14, Fig. 24–15). Transforminal lumbar interbody fusion with instrumentation can be performed safely and effectively by minimally invasive techniques, reducing significant morbidity and pain associated with conventional open techniques. They can accomplish all the surgical goals of the open procedures and thus are likely to replace the open techniques as the standard for lumbar interbody fusion. REFERENCES 1. Cloward RB. Posterior lumbar interbody fusion updated. Clin Orthop. 1985;193:16–19. 12. Wetzel FT, LaRocca H. The failed posterior lumbar interbody fusion. Spine. 1991;16:839–845. 15. Hutter C. Spinal stenosis and posterior lumbar interbody fusion. Clin Orthop. 1985;193:103–114. 16. Branch CL, Jr. The case for posterior lumbar interbody fusion. Clin Neurosurg. 1996;43:252–267.
24

Minimally Invasive Transforaminal Lumbar Interbody Fusion
















< div class='tao-gold-member'>
Minimally Invasive Transforaminal Lumbar Interbody Fusion
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








