leprosy, or sarcoidosis. Asymmetric neuropathy is also seen in multifocal motor neuropathy with conduction block, sometimes with increased anti-GM1 antibody titers, multifocal demyelinating sensory and motor neuropathy (Lewis-Sumner syndrome), and brachial neuritis.
roots form the lower trunk. The upper trunk gives off a small branch, the suprascapular nerve, which supplies the supra- and infraspinatus muscles. All trunks pass through the supraclavicular fossa under the cervical and scalene muscles. Each trunk then forms two branches and these branches regroup to form new divisions, the cords, as they course through the thoracic outlet, between the first rib and the clavicle, along with the subclavian artery. The lateral branches of the upper and middle trunks contribute to the lateral cord (i.e., C5, C6, C7), whereas the medial branches join with the lateral branch of the lower trunk and move dorsally to form the posterior cord (i.e., C5, C6, C7, C8). Finally, the lower trunk gives rise to the medial cord (i.e., C8, T1). The lateral and medial pectoral nerves branch off near the juncture of the trunks and the lateral and medial cords, respectively, supplying the pectoralis major muscle.
TABLE 87.1 Neuropathy Diagnosis and Laboratory Tests | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 87.2 American Academy of Neurology Recommendations for Testing for Distal Symmetric Polyneuropathy | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
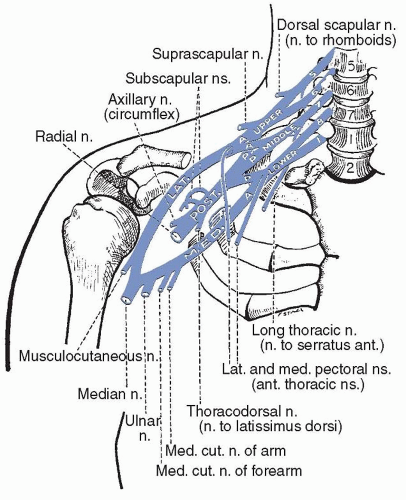 FIGURE 87.1 The brachial plexus. (From Haymaker W, Woodhall B. Peripheral Nerve Injuries. Philadelphia: WB Saunders; 1945.) |
TABLE 87.3 Innervation of the Muscles of the Shoulder Girdle | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 87.4 Innervation of Muscles of the Arm and Forearm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
neuropathy, or from multifocal motor neuropathy. A variety of insults may produce injury selectively affecting the brachial plexus, but tumor infiltration, radiation plexitis, and idiopathic plexitis are among the most important. Almost any neoplasm with a propensity for the chest may affect the plexus, but those cancers originating locally, such as lung and breast cancer, are most likely to cause injury. Such tumors may cause extrinsic compression of the plexus as they grow or may directly infiltrate the nervous tissue. Other neoplasms, such as lymphoma, may infiltrate the plexus and cause progressive deficits without any apparent mass effect or enlargement of the plexus itself in the initial stages. Magnetic resonance imaging (MRI) with contrast is the best way to confirm these lesions.
limb tension test” is said to be comparable to straight leg raising, is carried out by abducting the arms to 90 degrees in external rotation, bringing on symptoms within 60 seconds. EMG, MRI, and sonography after raising the arm have been used to aid in diagnosis. However, warnings continue to appear about false-positive maneuvers to evoke pain. Danielson and Odderson injected botulinum toxin into the anterior scalene muscle under ultrasound guidance. Subclavian artery flow rates were measured with Doppler ultrasound. Three weeks later, symptoms had improved and blood flow in the artery, tested with the arm extended, had improved. A placebocontrolled formal study of this “scalene muscle chemodenervation” would help resolve the uncertainty of the procedure which could be used for diagnosis, treatment, and screening for surgery. However, no consensus has been achieved for the surgical procedure of choice, which may or may not include botulinum, rib resection, brachial plexus neurolysis, scalenectomy, or release of the subclavian artery and vein. Exercise programs have also been advocated.
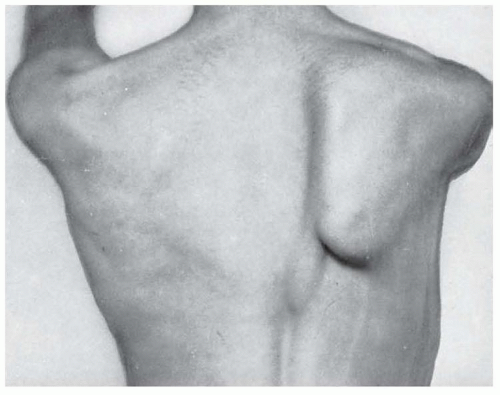
stretches and compresses it. Typically, such pressure is caused by carrying excessively heavy loads on the shoulder (e.g., furniture, carpets, heavy sacks, backpacks slung over one shoulder, etc.), although it may also appear after acute impact, such as that occurring while playing football. A more archaic term, although one still in use, is hod-carrier’s palsy, in reference to the hod or container bricklayers formerly placed on the shoulder to carry bricks up to roof tops when constructing chimneys. Injury of this nerve destabilizes the scapula, causing winging, and prevents the rotation of the scapula needed to enable the last few degrees of abduction of the arm from 90 to 180 degrees over the head. Injury following acute or chronic trauma is characterized by weakness in elevation of the arm above the horizontal plane. Winging of the scapula is most prominent when the arm is fully abducted or elevated anteriorly (Fig. 87.2). Winging is often not readily apparent with the arm resting at the side.
motor anterior interosseous nerve, which supplies the flexor pollicis longus, pronator quadratus, and flexor digitorum profundus I and II, and into a main branch, which passes through the carpal tunnel, further branching into the recurrent thenar nerve, supplying the abductor and the lateral flexor pollicis brevi and the opponens pollicis before terminating in the palm, where it supplies lumbricals I and II. Pronation is mediated by the pronator quadratus and pronator teres, wrist flexion by the flexor carpi radialis and palmaris longus, flexion of the thumb and the index and middle fingers by the superficial and deep flexors, and opposition of the thumb by the opponens pollicis (Table 87.6).
TABLE 87.5 Muscles Innervated by the Radial Nerve | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
TABLE 87.6 Muscles Innervated by the Median Nerve | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree