We have instruments of precision in increasing numbers with which we and our hospital assistants at untold expense make tests and take observations, the vast majority of which are but supplementary to, and as nothing compared with, the careful study of the patient by a keen observer using his eyes and ears and fingers and a few simple aids.
I. ARRAY OF NEURODIAGNOSTIC PROCEDURES
After completing the history and neurologic examination (NE) and proposing a tentative diagnosis, the examiner (Ex) has to decide whether further studies are required. Table 13-1 reviews the array of standard diagnostic tests. The goal is to choose the one or two safest, least invasive, and most economical procedures that will best confirm or refute the tentative diagnosis. Do not order every conceivable test to cover every diagnostic possibility. Go for the jugular. If you fail initially to secure the diagnosis, select successive tests in a logical order. In this section we will discuss the clinical use of lumbar puncture (LP) and neuroimaging studies.
TABLE 13-1 • Ancillary Neurodiagnostic Procedures
A. Neuroradiologic imaging 1. Plain films 2. CT (enhanced and unenhanced) 3. MRI (enhanced and unenhanced) 4. Angiography:MRA, direct injection, CTA, Doppler sonography 5. Cranial ultrasonography 6. Radionuclide scanning: PET, SPECT B. Electroneurodiagnosis 1. Electroencephalography (EEG) 2. Electromyography (EMG) 3. Nerve conduction velocity (NCV) 4. Evoked responses: visual, auditory, and somatosensory 5. Audiogram C. Punctures 1. Subarachnoid (LP or cisternal) for CSF 2. Subdural for blood, pus, or fluid 3. Ventricular for blood or CSF 4. Stereotaxic for lesion biopsy D. Blood biochemistry 1. Complete metabolic profile 2. Toxicology screen 3. Quantitative amino acids and organic acids 4. Serum enzymes 5. Long-chain fatty acids, cholesterol, cholestanol 6. Serum ceruloplasmin 7. B1, B12 and folic acid 8. Lysosomal enzymes 9. Collagen vascular screen 10. Disease-specific tests for inborn errors of metabolism 11. Hypercoagulable screen 12. Protein fractionation E. Neuro-ophthalmology 1. Visual acuity 2. Visual fields, central and peripheral 3. Ophthalmoscopy direct and indirect, slit-lamp inspection of media and fundus 4. Visual evoked response (VER) 5. Electroretinogram (ERG) 6. Electronystagmography 7. Optical coherence tomography (OCT) F. Urinalysis 1. Routine including specific gravity 2. Screen for inborn errors of metabolism 3. Toxicology screen 4. Catecholamines G. Neuropsychological tests 1. Developmental 2. IQ 3. Achievement 4. Neuropsychological battery 5. Personality profile H. Biopsy 1. Muscle 2. Nerve 3. Skin 4. Brain I. Microbiologic tests 1. Serologic 2. Culture of microorganisms 3. Immunologic screen, T and B cells, blood titers, γ-globulin 4. Polymerase chain reaction J. Genetic tests 1. Karyotype 2. Genotype |
ABBREVIATIONS: CSF = cerebrospinal fluid; CT = computed tomography; CTA = Cranial computed tomography angiography; LP = lumbar puncture; MRA = magnetic resonance angiography; MRI = magnetic resonance imaging; PET = positron emission tomography; SPECT = single-photon emission computed tomography. |
II. THE CEREBROSPINAL FLUID EXAMINATION
A. Location, origin, and circulation of the cerebrospinal fluid
1. Examination of the cerebrospinal fluid (CSF) dates to Heinrich Iraneous Quincke who introduced spinal puncture in 1891 for the treatment of hydrocephalus.
2. Location of the CSF: The CSF occupies the ventricles and subarachnoid spaces. The total volume of CSF is about 150 mL. The subarachnoid space separates the arachnoid membrane from the pia mater. In addition to the CSF, the subarachnoid space contains the blood vessels that enter or leave the central nervous system (CNS).
3. Formation of the CSF
a. The CSF is formed by:
i. The choroid plexuses of the lateral, III, and IV ventricles.
ii. Water produced by oxidative metabolism.
iii. An ultrafiltrate through the blood–brain barrier of the cerebral capillaries (Fishman, 1992; May et al, 1990).
b. The rate of production is 0.3 to 0.4 mL/min, or about 500 mL/day.
4. Circulation of the CSF
a. The CSF exits the ventricles by flowing out of the foramina into the IV ventricle, the Lateral foramina of Luschka and the Median foramen of Magendie. The ependyma lining the ventricles absorb some CSF (Rando and Fishman, 1992). Learn Fig. 13-1.
b. After exiting from the foramina of the IV ventricle, the CSF enters the subarachnoid space. It may then percolate downward around the spinal cord or upward over the cerebral hemispheres to the superior sagittal sinus.
i. Pacchionian granulations that extend from the subarachnoid space into the lumen of the sinus allow absorption of the CSF into the venous blood (Fig 13-2).
ii. At the spinal level, drainage takes place at the root sleeves, where the pia and arachnoid fuse with the connective tissue of the spinal nerves.
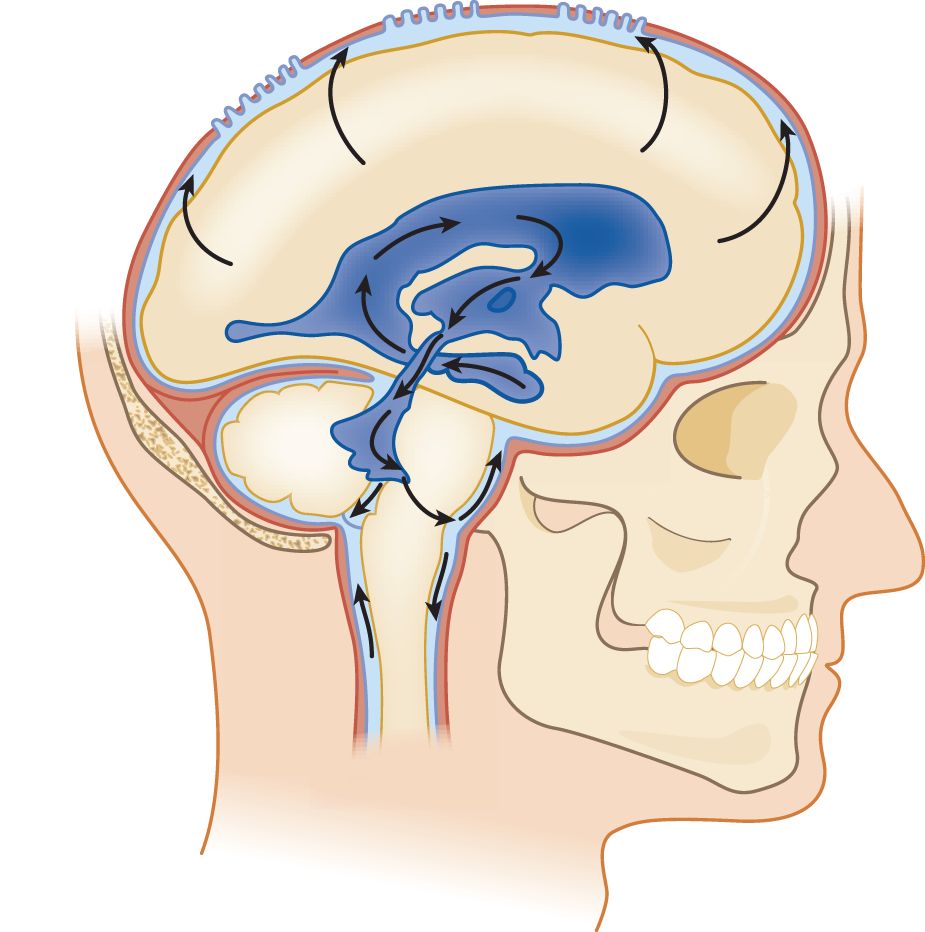
FIGURE 13-1. Lateral view of the ventricular system and CSF circulation. Beginning in the temporal horn, trace a drop of CSF through the III ventricle, aqueduct, out the IV ventricle, into the subarachnoid space, and up over the convexity of the hemisphere to the Pacchionian granulations along the superior sagittal sinus. CSF = cerebrospinal fluid.
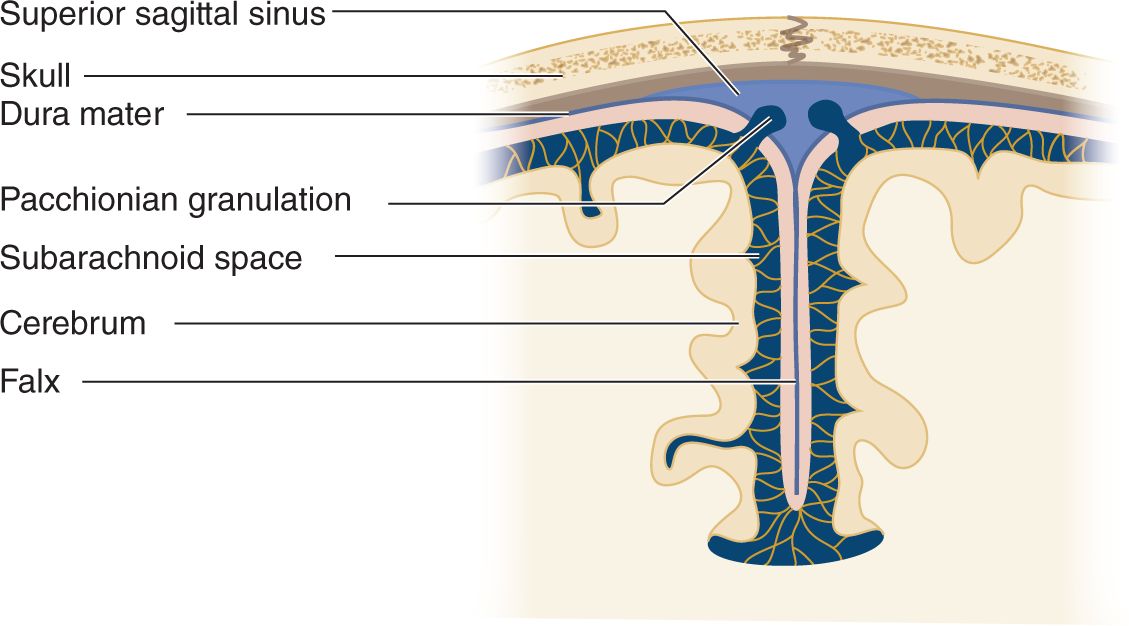
FIGURE 13-2. Coronal section through the cerebral falx to show the Pacchionian granulations projecting directly into the superior sagittal sinus. The CSF passes through the granulations into the venous blood of the sinus. CSF = cerebrospinal fluid.
5. Trace a drop of CSF from the temporal horn of the lateral ventricle to its absorption into the blood.
_________
_________
B. Functions of the cerebrospinal fluid
1. The CSF in the subarachnoid space provides a flotation layer around the brain and spinal cord that cushions them from trauma.
2. The CSF permits circulation of metabolites and electrolytes, neurotransmitters, peptide hormones, antibodies, leukocytes, and various other normal and abnormal cells.
3. The CSF aids in regulating the pH and electrolyte balance of the extracellular space of the CNS.
C. Composition of the cerebrospinal fluid
The CSF is a sparkling clear salt solution containing a few white blood cells (WBCs), proteins, sugar, traces of enzymes, neurohumors, neurotransmitters, and other metabolites (Fishman, 1992). The cell count, sugar, and protein levels change with age (see the top rows of Table 13-2 and Fig. 13-3) (Video 13-1).
TABLE 13-2 • Typical Cerebrospinal Fluid Profiles in Normal Individuals and in Various Diseases
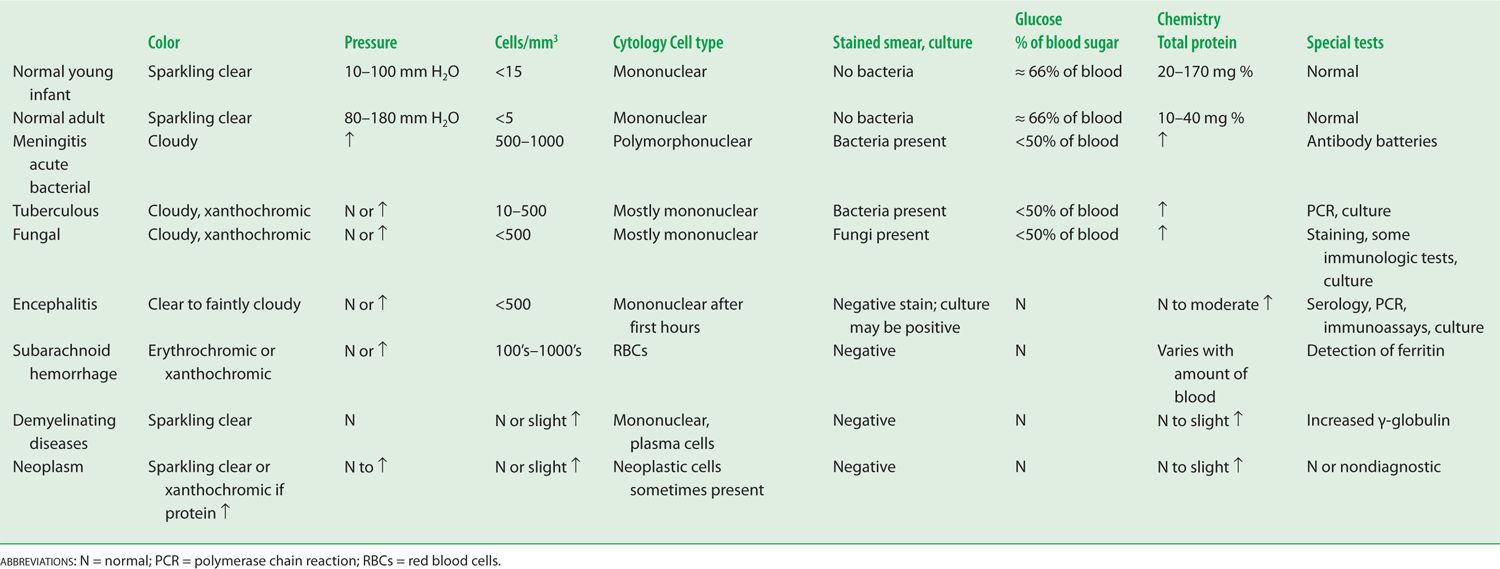
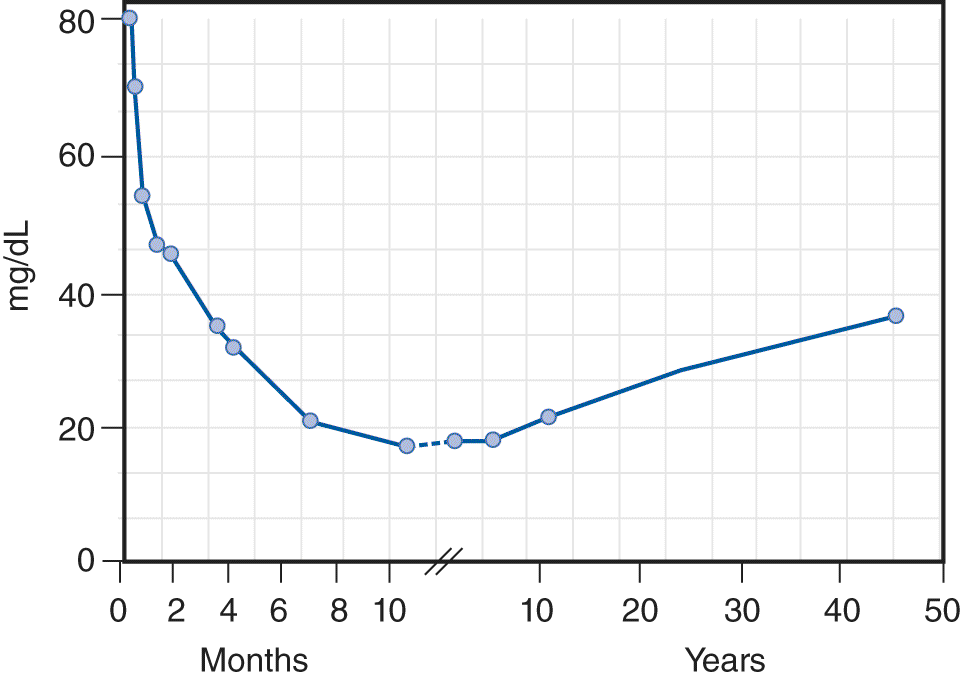
FIGURE 13-3. Age-related changes in the average total protein content of the cerebrospinal fluid. (Reproduced with permission from Widell S. On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo-encephalitis. Acta Paediatr. 1958 November;47(6):711–713.)

Video 13-1. Patient with HIV and aseptic meningitis attributed to immune reconstitution inflammatory syndrome (IRIS).
D. Normal pressure of the cerebrospinal fluid
Measure the pressure of the CSF by attaching a manometer to a needle inserted into the subarachnoid space (Fig. 13-4A). The normal CSF pressure depends on the patient’s (Pt’s) age (Table 13-2): 10 to 100 mm of water for young infants, 80 to 180 mm of water for normal mature individuals, and up to 250 mm of water for grossly obese individuals, perhaps because of increased intra-abdominal pressure (Whiteley et al, 2006). Diseases may increase or decrease intracranial pressure. Increased intracranial pressure is by far the most common problem.
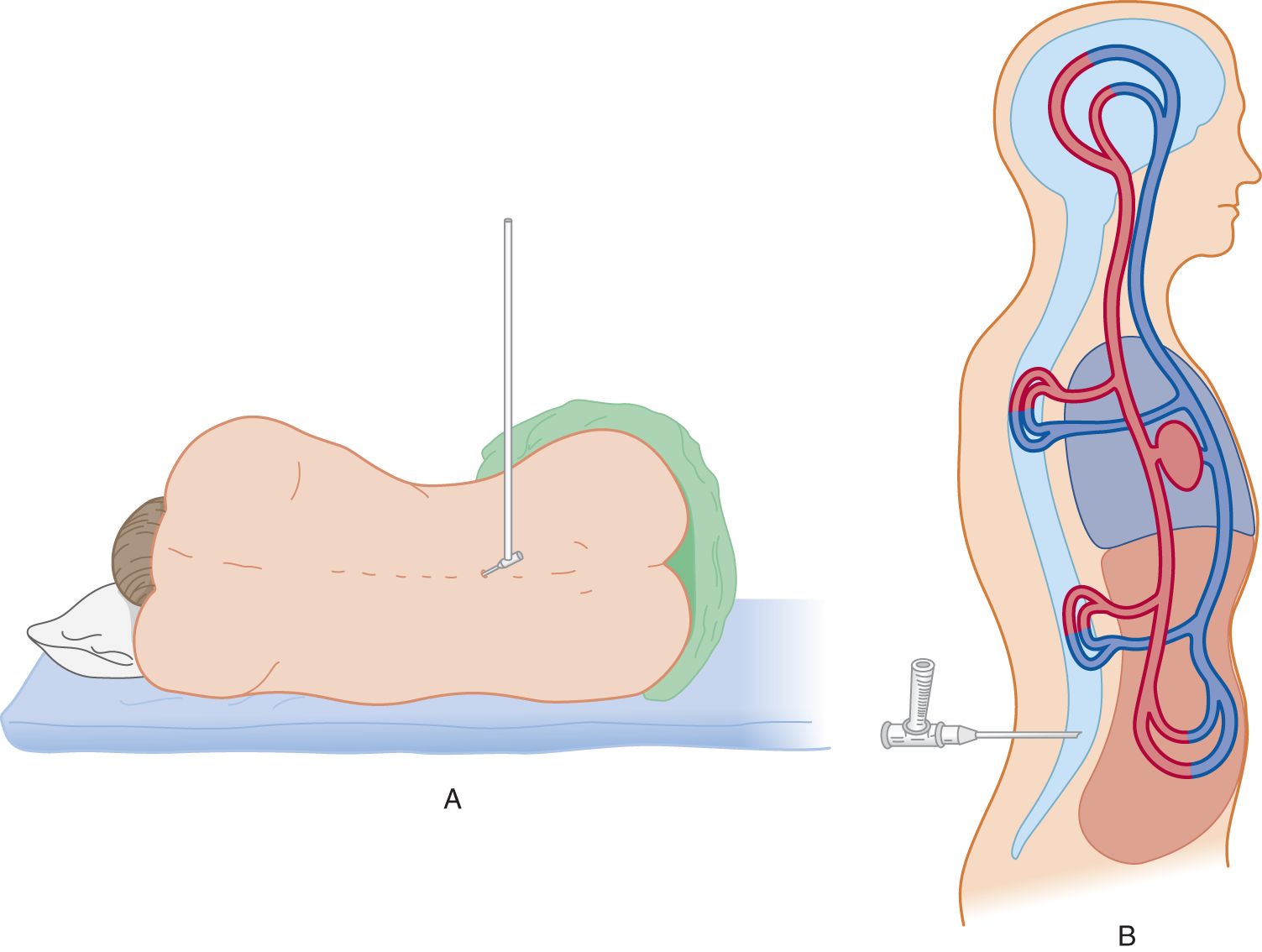
FIGURE 13-4. (A) Patient in left lateral position with legs and back flexed. A manometer is attached to a needle inserted into the subarachnoid space. (B) Top view of patient diagrammatically representing the continuity of vessels inside and outside the craniovertebral cavity.
E. Increased intracranial pressure
1. Symptoms of increased intracranial pressure: Symptoms consist of headaches, nausea and vomiting, dizziness, transient blurring of vision or blindness (transient obscurations of vision), and mild obtundation. Mothers of children who have shunts to treat increased pressure often can tell by overall changes in the child’s behavior that the shunt is blocked or malfunctioning.
2. Signs of increased intracranial pressure
a. Increased pressure in infants causes a bulging fontanel, split sutures, and an increasing occipitofrontal circumference that moves successively upward on the percentile lines of an occipitofrontal circumference chart (Fig. 1-23).
b. In older Pts, papilledema occurs and often a VI nerve palsy develops as a consequence of compression of the nerve during its long intracranial course.
3. Causes of increased intracranial pressure
a. Expanding lesions within the craniovertebral space, such as hematomas, neoplasms, abscesses, and brain edema. Rarely, increased production of CSF may cause increased pressure.
b. Prolonged status epilepticus or hypoxia, which causes brain edema.
c. Metabolic encephalopathies: Hepatic, uremic, Reye syndrome, idiopathic intracranial hypertension (pseudotumor cerebri), and endocrinopathies.
d. CNS infections: Meningitis and encephalitis may incite extreme edema and increased pressure.
e. Obstructive lesions that impede the flow of CSF from the ventricles to the subarachnoid space and through the Pacchionian granulations. The most common sites of blockage are
i. The interventricular foramen of Monro, usually by a neoplasm.
ii. The cerebral aqueduct, usually by congenital atresia or stenosis, inflammatory adhesions, or neoplastic compression.
iii. The IV ventricle and its outlets, usually by posterior fossa neoplasms, inflammatory adhesions or failure of outlets to perforate, as in the Dandy–Walker malformation.
iv. The subarachnoid space, usually by adhesions after meningitis or subarachnoid hemorrhage.
v. The Pacchionian granulations, because of clogging of the granulations (and root sleeves) by blood or extremely high CSF protein.
vi. The intracranial venous sinuses, because of thrombosis (Video 13-2 CVT).

Video 13-2. Patient with advanced bilateral papilledema due to superior sagittal sinus venous thrombosis.
4. Increased intracranial pressure and Pascal law
a. Physically, the CSF is essentially water and the CNS is about 80% water. Students who have handled only the stiff, formalin-fixed brain of the cadaver do not appreciate the supple softness of the living brain. Its compliancy resembles a foam sponge pillow or a balloon (the pia-arachnoid) filled with molasses. Therefore, a physicist, in studying intracranial hydrodynamics, might represent the CNS and the CSF as a single homogeneous fluid. To duplicate biologic conditions, the combined CNS–CSF model includes the vascular space within the craniovertebral space, as shown in Fig. 13-4B.
b. The CNS–CSF in the craniovertebral space is itself incompressible, like any fluid. According to Pascal law, pressure exerted on a fluid in a closed container is transmitted equally in all directions. The pressure transmission is independent of the size or shape of the container. Therefore, pressure in the lumbar CSF reflects the intracranial pressure, although biological factors alter the simple expression of Pascal law.
c. As intracranial pressure increases, it displaces CSF and the intravascular blood (Fig. 12-9). If these compensations fail, any further increase in pressure causes the brain to herniate down through the foramen magnum, the only place it can go, the only way out of the brain case (Chapter 12).
F. Low cerebrospinal fluid (CSF) volume (pressure) syndrome
1. Symptoms and signs: The Pt experiences orthostatic headache, vertigo, tinnitus, nausea, and vomiting, especially when rising from a reclining to a vertical position, and may faint. The symptoms could occur because of loss of flotation, allowing the cerebrum to sag onto the brainstem. A variety of connective tissue disorders have been associated with the development of this syndrome (Schievink, 2006).
2. Causes of low CSF pressure
a. Medical procedures that pierce the meninges and establish a CSF leak include lumbar puncture and neurosurgical operations.
b. Basal skull fractures that create fistulae in the nose or middle ear, causing CSF rhinorrhea or otorrhea (Video 13-3).
c. Severe dehydration.
d. Leakage along nerve roots (Rando and Fishman, 1992).
e. Idiopathic aliquorrhea (Rando and Fishman, 1992).

Video 13-3. Cranial fossa fracture in a patient with bilateral raccoon’s eyes and a right Battle sign describing CSF otorrhea. The patient also exhibits asterixis of the upper extremities. CT also demonstrates pneumocephalus.
G. Indications for lumbar puncture (spinal puncture or spinal tap)
1. To identify infections of the CSF. Repeated lumbar punctures serve to monitor the effect of treatment.
2. To identify subarachnoid bleeding.
3. To identify neoplastic invasion or seeding of the subarachnoid space by gliomas, carcinomas, or leukemias and lymphomas.
4. To measure and fractionate CSF proteins in suspected immunologic diseases, especially multiple sclerosis, and for the differential diagnosis of some neuropathies, such as Guillain–Barré syndrome (albuminocytologic dissociation).
5. To measure pH, electrolytes, enzymes, neurotransmitters, and trace constituents in the diagnosis of the genetic/metabolic encephalopathies (Hyland and Arnold, 1999).
6. To introduce chemotherapeutic or antibacterial agents or anesthetics.
7. To introduce contrast agents for myelography or radionuclides for study of CSF flow dynamics and to locate leaks.
8. To measure for increased CSF in patients with possible pseudotumor cerebri (idiopathic intracranial hypertension). In such cases, imaging studies have excluded a mass lesion that would cause herniation. An LP is also required to document low CSF pressure in low-pressure syndromes.
H. Contraindications to a lumbar puncture
1. Infection of the lumbar skin or deeper tissues through which the needle must pass.
2. Coagulopathies: Spinal subarachnoid hemorrhage or spinal subdural hematomas may follow an LP in these Pts (Masdeu et al, 1979). Diseases, such as hemophilia and thrombocytopenia, or anticoagulant therapy represent relative rather than absolute contraindications.
3. Cervical cord lesions: Removal of CSF from the lumbar region may cause the cord to shift against the lesion, resulting in quadriplegia, apnea, and death.
4. Increased intracranial pressure from a suspected or known intracranial mass lesion.
a. If the history, NE, or ophthalmoscopic examination suggest a mass lesion or increased intracranial pressure, an LP generally should not be done. The presence of distinct venous pulsations virtually excludes increased intracranial pressure. Nearly 90% of normal persons show pulsations if both eyes are carefully examined (Levin, 1978). The absence of venous pulsations does not establish increased pressure because some normal Pts do not show such pulsations.
b. The most common mass lesions that cause brain herniation include neoplasms, hematomas, abscesses, cerebral edema, and massive cerebral or cerebellar hemispheric infarction or hemorrhage. Of 22 Pts with brain abscess, five showed evidence of herniation within 2 hours of an LP (Samson and Clark, 1973). Do not perform LPs in Pts with brain abscess. The diagnosis depends on radiographic imaging findings.
c. Posterior fossa lesions, which may cause transforaminal herniation, also pose a great threat. Signs of impending transforaminal herniation include a stiff neck, hiccups, irregular breathing, apnea, hypotension, and quadriparesis (Hartmann et al, 1994). Note also that posterior fossa masses may cause upward herniation out of the posterior fossa, thereby compressing the midbrain. If the clinical findings raise any question of a mass lesion that may cause transtentorial or transforaminal or upward herniation, always order head computed tomography (CT) or, better, brain magnetic resonance imaging (MRI) before doing an LP. The radiographic examination may clinch the diagnosis and render the LP useless and potentially harmful (Hasbun et al, 2001).
d. Sometimes clinical exigencies will call for an LP even in the presence of increased pressure, to identify or exclude a specifically treatable disorder. Conditions with possible increased pressure that may require an LP include suspected meningitis or encephalitis and idiopathic intracranial hypertension. Then the Ex has to weigh the indications against the contraindications (Fishman, 1992). In these cases, consider pretreating the Pt with mannitol and hyperventilation to reduce intracranial pressure before the LP.
e. In the management of increased pressure from acute head injuries and other emergencies and coma, neurosurgeons implant a pressure transducer within the skull for continuous monitoring of the results of treatment.
I. Complications of a lumbar puncture
1. Transtentorial or transforaminal herniation of the brain, causing death. Typically, this occurs within 2 hours of the LP.
2. Back pain at the puncture site.
3. Bleeding: Epidural, subdural, or subarachnoid; a so-called bloody tap (Masdeu et al, 1979). Spinal epidural hematoma is a rare and devastating condition which may be due to trauma, surgery, epidural catheterization, coagulation disorders, anticoagulation therapy, arteriovenous malformations, cavernous malformations, and Paget disease. Thrombocytopenia or other bleeding diathesis or systemic heparin or warfarin administration increase the risk of puncture-induced spinal canal hemorrhage (epidural, subdural or subarachnoid). Spinal epidural hematoma or spinal hematoma after neuraxial anesthesia is a rare complication with anticoagulation therapy (Wysowski et al, 1998). A spinal epidural hematoma may produce progressive radicular pain and sphincter disturbances, and represents a neurological emergency requiring urgent investigation and treatment. Most neurologic sequelae seem to relate to inadvertent insertion of the needle at too high a level (Hamandi et al, 2002). Thus, strict application of guidelines for performing spinal procedures in anticoagulated patient is recommended (Horlocker et al, 2003)
4. Headache and post-LP low CSF volume (pressure) syndrome: See Section F. Most Pts have some backache. Up to 32% (wide range of reported frequency) have headache after an LP (Armon and Evans, 2005). The use of atraumatic needles has reduced the incidence of post LP headache to about 3% (Lavi et al, 2006). Low CSF pressure from CSF leakage presumably causes post-LP headache.
a. The headache begins a day or two after the LP, is often throbbing, mainly occurs in the upright position, is worsened by coughing or straining, and improves when the Pt reclines. The Pt may have a stiff neck, nausea and vomiting (Kuntz et al, 1992).
b. Most post-LP headaches resolve spontaneously by approximately 10 to 14 days. Bed rest (none, 2-8 hours, or up to 48 hours), forcing fluids, or intravenous caffeine are of questionable benefit in helping to prevent post-LP headaches (Roos, 2003a).
c. Refractory headaches respond to an epidural injection of 15 to 20 mL of autologous blood at the puncture site (Sencakova et al, 2001), presumably because it seals the puncture wound (Boonmak and Boonmak, 2010).
5. Double vision, which usually results from unilateral or bilateral cranial nerve VI palsies is usually self-limited.
6. Iatrogenic infection: very rare.
7. Implantation of epidermoid tumors in the lumbosacral canal: a very rare complication (Park et al, 2003).
8. In summary, the three most dangerous circumstances for an LP are increased intracranial pressure, a mass lesion capable of producing herniation, and a high cervical cord lesion.
J. Preparation for a lumbar puncture
1. Always get an MRI or CT scan whenever the Pt has the new onset of overt neurologic signs and symptoms, an altered consciousness, or seizures (Hasbun et al, 2001).
2. Always get an MRI if the Pt might have a compressive spinal cord lesion.
3. Ensure that the radiographs are competently read, particularly with respect to ventricular size, presence of absence of sulci and cisterns, and the absence of a posterior fossa lesion. MRI is usually superior to CT, except for emergency screening for large mass lesions, edema, or recent bleeding.
4. Review the history and medical record for a bleeding tendency. The platelet count should exceed 50,000 and the international normalized ratio (INR) should be less than 1.2. While preprocedural administration of aspirin therapy has not been associated with a high risk of bleeding with LP or other spinal anesthetic interventions, data on clopidogrel are lacking (Horlocker et al, 1995; Doherty and Forbes, 2014).
5. Decide whether to send a simultaneous blood sample for comparison with the CSF with respect to chemistry and rising antibody titers.
6. Psychological preparation of the Pt for an LP: Everyone dreads needles, particularly a stab in the back. Every layperson, it seems, knows of someone who had one of “those taps” and afterward had a permanent backache or never walked again. Of course, that conclusion has reversed cause and effect: the LP was done because of the disability, but litigious Pts today blame the doctor, not the disease. The Ex must ensure that the indications are valid and that the Pt understands the reasons for the procedure. Because an LP counts as surgery, have the Pt sign a consent form listing complications such as backache, headache, infection, and bleeding.
K. Technique for lumbar puncture
1. Positioning of the Pt for an LP
a. The Pt assumes the lateral recumbent position with the head, spine, and extremities flexed (the fetal position; Fig. 13-4A). A pillow under the head keeps it aligned with the spine. In the trunk-flexed position, the distance between the dorsal processes and lamina of adjacent vertebrae  increases/
increases/ decreases. (
decreases. ( increases) (Spinal flexion thus increases the target area for the needle. Review Fig. 12-24 if you erred.)
increases) (Spinal flexion thus increases the target area for the needle. Review Fig. 12-24 if you erred.)
b. Cooperative and not acutely ill Pts may sit with the spine flexed. However, in this position, the measurement of the intraspinal pressure is unreliable. If the pressure reading is important (eg, in a patient with possible pseudotumor cerebri), the lateral recumbent position is preferred.
2. Needle insertion and manometry for measuring CSF pressure
a. Clean the lower lumbar area with Betadine or 70% alcohol and apply a sterile drape.
b. Select the vertebral interspace, between L3 and L4, between L4 and L5, or between L5 and S1, by palpation or by lining up the vertebral interspace with the top of the iliac crests, which is at the interspace between L3 and L4. Insertion of the needle rostral to that level may damage the distal tip of the spinal cord, which usually ends at L2 (Fishman, 1992).
c. Depending on the Pt, anesthetize the insertion point with 1% lidocaine.
d. Insert a 20- to 22-gauge needle, with its stylet in place, into the interspace between the dorsal processes of the vertebrae at the selected level. Angle the needle slightly cephalad to parallel the slant of the dorsal spines of the vertebrae (Fig. 12-24). Insert the needle until a slight “pop” is felt as the needle pierces the dura and enters the subarachnoid space. The larger the bore of the needle, the easier it is to reach the subarachnoid space, but the greater the leakage of fluid after withdrawal of the needle.
i. Insert the needle with the bevel of the needle turned parallel to the long axis of the spine (Roos, 2003b). The bevel then is thought to more cleanly separate rather than transect the longitudinal fibers of the dura, thus reducing post-LP leakage of CSF. A problem with the theory is that dural fibers run in a variety of directions, not just longitudinally.
ii. To reduce post-LP leakage and post-LP headaches, Whitacre or Sprotte dull-tipped needles have been advocated to replace the standard sharp-tipped Quincke needle (Strupp et al, 2001). These needles have a blunt tip alleged to separate rather than cut the dural fibers. The needles drain CSF through an oval opening on the lateral aspect of the shaft, just proximal to the blunt tip, rather than through the tip itself (Evans et al, 2000).
iii. Before insertion of a blunt-tipped needle, the Ex uses a sharp-tipped “introducer” needle to cut a path about two-thirds of the way in.
e. After feeling the initial pop, the Ex withdraws the stylet. A drop of CSF should appear at the hub of the three-way stopcock on the LP needle. Attach a manometer and allow the fluid level to stabilize in the manometer. Record the opening pressure. At the end of the procedure, record the closing pressure.
f. If the history or ophthalmoscopic examination raises a concern about increased pressure, proceed this way. Insert a needle through the skin but stop it just short of the subarachnoid space. Remove the stylet and attach a manometer. With the manometer in place and with a fingertip blocking the open bore at the top of the manometer, advance the needle into the subarachnoid space and allow the fluid to fill the manometer gradually. Otherwise, the Ex may insert the needle with its regular stylet in place, withdraw it, and quickly attach a three-way stopcock and manometer.
g. For premature and young infants, use a standard butterfly needle (Greensher et al, 1971). The Ex should limit the depth of any needle puncture to 2.5 cm in these infants.
3. Fluctuations in the meniscus in the manometer
a. The meniscus of CSF in the manometer will fluctuate around some mean value, for example, 120 mm of water (Fig. 13-5).
b. Causes for fluctuations: As shown in Fig. 13-4B, the intracranial and intraspinal veins communicate directly with the extracranial and extravertebral veins. Because these communications lack valves, the extracranial veins transmit any change in their pressure directly into the craniovertebral space, in particular the changes in intrathoracic and intra-abdominal pressure (Whiteley et al, 2006). When a person inhales air, the CNS–CSF pressure  increases/
increases/ decreases/
decreases/ does not change. (
does not change. ( decreases) (The intrathoracic pressure decreases with inspiration. Inspiration sucks blood from the CNS veins and the CNS-CSF pressure drops. Breathing causes rhythmic excursions of the manometer meniscus at the rate of 16 per minute, as shown in Fig. 13-5.)
decreases) (The intrathoracic pressure decreases with inspiration. Inspiration sucks blood from the CNS veins and the CNS-CSF pressure drops. Breathing causes rhythmic excursions of the manometer meniscus at the rate of 16 per minute, as shown in Fig. 13-5.)
c. Contraction of the abdominal muscles would  increase/
increase/ decrease the intracranial pressure. Explain.
decrease the intracranial pressure. Explain.
_________
_________ increase) (The intervertebral veins reflect the increased intra-abdominal pressure backward into the craniovertebral space, thus reducing the outflow of blood from the CNS. Because the arterial input continues, the CNS–CSF pressure increases.)
increase) (The intervertebral veins reflect the increased intra-abdominal pressure backward into the craniovertebral space, thus reducing the outflow of blood from the CNS. Because the arterial input continues, the CNS–CSF pressure increases.)
d. The pulse causes smaller excursions of the meniscus (not shown in Fig. 13-5 because of their small amplitude). Because the arterial walls absorb much of the arterial pressure, the CNS–CSF pressure most closely reflects the capillary and venous pressures.
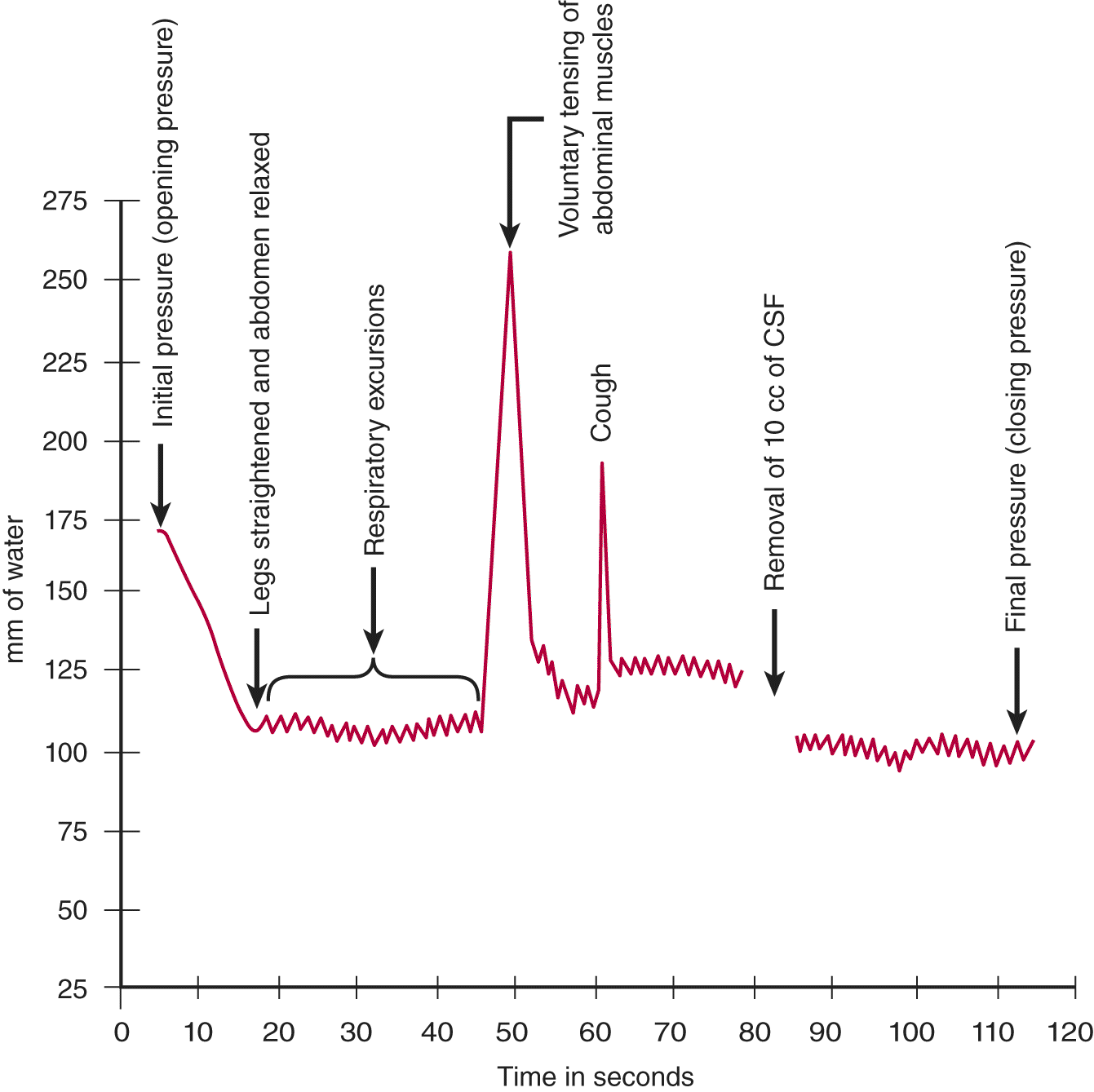
FIGURE 13-5. Graph of cerebrospinal fluid pressure, as measured by manometry. Starting at the left, read through the legends just above the arrows.
L. Clinical evaluation of increased pressure as registered by manometry
1. Assume that the opening pressure in the manometer is 240 mm of water, which is  normal/
normal/ low/
low/ high. (
high. ( high)
high)
2. Two explanations exist:
a. The intrinsic CNS–CSF pressure is too high.
b. The pressure reflects factors extrinsic to the craniovertebral space.
3. An LP always makes the Pt anxious and causes increased tension in the skeletal muscles. What, then, is a simple extrinsic cause for increased CNS-CSF pressure? _________
4. Encourage the Pt to relax the flexed position and to take a few deep breaths. In the Pt with the opening pressure of 240 mm of water, these maneuvers dropped the pressure to 210 mm of water. This value is  normal/
normal/ low/
low/ high. (
high. ( high)
high)
5. The flexed posture has caused the Pt to flex the head on the chest, and it may have bent somewhat to the side while resting on the pillow. Because flexion or turning of the head may compress the jugular veins, the Ex straightened the Pt’s head and readjusted it on the pillow. These maneuvers failed to drop the pressure below 200 mm of water, indicating that the Pt had slightly increased intrinsic intracranial pressure.
6. If an expanding intracranial lesion raises the CNS–CSF pressure, visualize what might happen after removal of CSF from the lumbar region (Fig. 13-6).
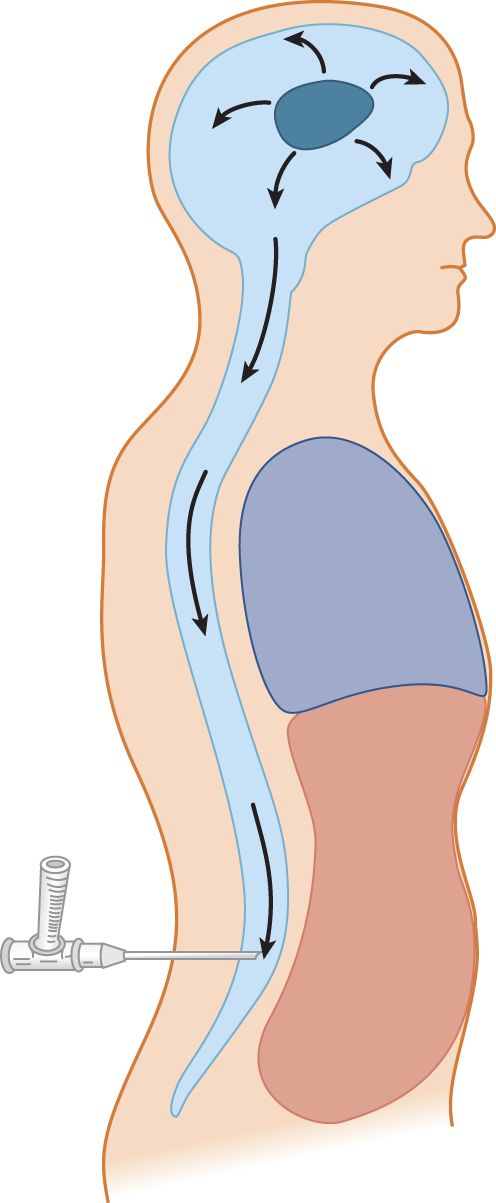
FIGURE 13-6. Depiction of an expanding intracranial lesion (oval black mass) causing increased intracranial pressure. The pressure exerts itself equally in all directions. Escape of cerebrospinal fluid through a lumbar needle allows the intracranial contents to flow (herniate) toward the region of lowered pressure.
7. Increased intracranial pressure may have caused impending herniation of the brain. The system may be delicately balanced, with the uncus and parahippocampal gyrus poised ready to plunge over the edge of the tentorium, or the cerebellar tonsils may be ready to herniate through the foramen magnum. These two potentially fatal herniations are called _________
8. Withdrawal of fluid from the lumbar region will allow the brain to flow down toward the point of low pressure, according to Pascal law. The potential herniation is converted to actual herniation. The Pt will _________
9. If, after careful measurement, the Ex concludes that the Pt truly has intrinsic increased intracranial pressure, what is the next step?  Withdraw fluid rapidly./
Withdraw fluid rapidly./ Withdraw fluid cautiously./
Withdraw fluid cautiously./ Withdraw the needle immediately! (
Withdraw the needle immediately! ( Withdraw the needle immediately!) (The small amount of fluid in the manometer suffices for a cell count or to see blood in the CSF, which is often the most important information needed.)
Withdraw the needle immediately!) (The small amount of fluid in the manometer suffices for a cell count or to see blood in the CSF, which is often the most important information needed.)
10. Describe the safe maneuvers used to exclude extrinsic factors as the cause for high spinal fluid pressure._________
M. Cessation of cerebrospinal fluid flow
1. Frequently, the flow of fluid starts but stops. Very rarely, transforaminal herniation, like a plug in a drain, blocks the transmission of intracranial pressure after a small amount of CSF has drained out. In this case, the Pt shows dramatic signs of apnea and quadriplegia. Most commonly, the cause is far more benign: Something has blocked the needle (Table 13-3).
TABLE 13-3 • Common Causes for Cessation of Cerebrospinal Fluid Flow and Their Remedies
Cause | Remedy |
Blood clot in the needle lumen | Replace stylet to ream out the needle |
Nerve root has fallen over the bevel of the needle | Rotate the shaft of the needle |
Displacement of the tip of the needle from the subarachnoid space or incomplete penetration | If you think the needle tip is too deep, withdraw it slightly; if too shallow, insert it farther |
2. After trying the maneuvers in Table 13-3, reattach the manometer to measure the pressure again. If the system is open from the subarachnoid space through the needle to the manometer, the manometer meniscus should show excursions at the rate of 16 per minute, caused by _________
3. The CSF may also stop flowing through the needle if a lesion occludes the vertebral canal.
4. What not to do if CSF stops flowing through the needle: Exasperation may tempt the Ex to try to suck CSF out with a syringe. If at this point you do not understand the dangers, this text has totally failed.
N. Collection and appearance of the cerebrospinal fluid
1. After measuring the opening pressure, collect 10 to 15 mL of CSF by allowing several milliliters to drip into each of three or four tubes. The normal fluid appears sparkling clear in all tubes. Inspect all tubes for cloudiness, redness (erythrochromia), and yellowness (xanthochromia). The attending physician, not the laboratory technician, bears the responsibility for proper inspection of CSF after collection.
2. To end the LP, measure the closing pressure and replace the stylet before withdrawing the needle. Replacing the stylet is thought to keep arachnoid strands from being sucked through the dural puncture site, which might prolong CSF leakage.
O. Cloudiness of the cerebrospinal fluid
1. Cloudiness usually means an increased number of WBCs in the CSF. Rarely, numerous bacteria cause cloudiness. Normally, the CSF contains five or fewer WBCs per mm3. More than 300 polymorphonuclear WBCs/mm3 or more than 400 to 500/mm3 of lymphocytes or monocytes will cause detectable cloudiness, if the Ex inspects the CSF properly. Obvious cloudiness occurs with counts of 600 to 800 WBCs/mm3.
2. To detect minimal cloudiness with counts in the range of several hundred WBCs per cubic millimeter, do the following:
a. Obtain an exact duplicate of the tube used to collect the CSF and add the same amount of water as is in the CSF tube. The duplicate tube must have the same translucency, color, and refractive index as the CSF tube.
b. Hold the duplicate and CSF tubes side-by-side against a white sheet of paper, against a dark background, and then against a light source. Use daylight, if at all possible. Compare the CSF with the water by looking through the sides of the tubes, as in using a colorimeter. The direct, side-by-side comparison enables the Ex to detect very subtle cloudiness or color changes.
c. In bright sunlight, even mild CSF pleocytosis with WBCs or red blood cells (RBCs) can cause the Tyndall effect, a snowy iridescence.
d. Students may suppose that this is all unnecessary because the CSF goes to the laboratory for the “official” examination and cell count anyway, so why bother? Well, the careful physician inspects the CSF personally and does the cell count on the spot, before the cells have undergone the autolysis that commences in an hour, and to gain the information quickly and reliably. In the second place, personal inspection checks the accuracy of the laboratory, a check that unfortunately is necessary. If the laboratory reports 20 WBCs/mm3 after the Ex has seen a cloudy fluid, the laboratory must be wrong. If the Pt has meningitis, you must avoid an error on this critical point.
P. Bloody taps, erythrochromia, and xanthochromia
1. RBCs get into the CSF at one of two times in relation to the tap:
a. From preexisting bleeding caused by a ruptured blood vessel or other CNS lesion.
b. From inadvertent bleeding caused by the needle puncture, a “traumatic” or “bloody tap.”
2. Frank bleeding causes a red CSF, called erythrochromia. It takes an RBC count of 100 to 300/mL to cause erythrochromia. Normally the RBC count in the CSF is _______. (zero)
3. In contrast to erythrochromia, a yellowish CSF is called xanthochromia.
4. Differentiation of preexisting bleeding from a bloody tap: A traumatic tap is generally inconsequential, whereas preexisting bleeding may foretell a life-threatening lesion. Three tests distinguish the two sources of RBCs, the four-tube test, centrifugation for xanthochromia, and cytologic demonstration of erythrophagocytosis by monocytes.
a. The four-tube test: Compare the redness of the successive tubes. Blood that enters the CSF before the tap will have mixed freely with the CSF. All tubes display the same color and will have the same cell count. Blood from a traumatic tap tends to clear in successive tubes.
b. Centrifugation for xanthochromia: If the tubes are uniform in color, centrifuge one to deposit the RBCs on the bottom and compare the supernatant fluid with a control tube, as described above. If the CSF is completely colorless, the RBCs entered the CSF recently, either less than 2 to 4 hours ago or from the tap itself. Discoloration of the supernatant fluid means that the RBCs entered more than 2 to 4 hours before and have undergone lysis. Free hemoglobin in solution causes the xanthochromia. If the bleeding occurred days before the tap, degradation products of hemoglobin, methemoglobin, and bilirubin cause the color. Because RBCs undergo crenation in the CSF, crenation does not signify preexisting blood.
c. Erythrophagocytosis: Cytologic examination proves preexisting bleeding by demonstrating phagocytosed RBCs in macrophages, but it takes many hours to days to develop.
5. Correction of the WBC for blood contamination: Subtract 1 WBC/mm3 for each 700 RBCs/mm3. The remainder approximates the true CSF WBC count.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


