20
Approaches and Management of Basilar Tip Aneurysms
Aneurysms of the basilar artery apex carry the highest natural history risk of rupture of all aneurysm locations. In the International Study of Unruptured Intracranial Aneurysms Update, the basilar apex location was the strongest predictor of hemorrhage (relative risk 2.3; 95% confidence interval 1.1–4.8; p = .025).1 In that same study, the posterior circulation location was a significant predictor of a worse clinical outcome after surgical and endovascular treatment (for surgery: relative risk 1.6; 95% confidence interval 1.1–2.4; p = .025; for endovascular treatment: relative risk 2.25; 95% confidence interval 1.1–4.4; p = .02).1 Others have also described the challenging nature of basilar tip aneurysm surgery.2 Thus, the cerebrovascular surgeon is faced with an aneurysm that has the highest risk of hemorrhage of all aneurysm locations if left untreated, but also the highest risk of morbidity associated with treatment.
♦ Combined Neurovascular Team Approach
At the Massachusetts General Hospital, we have adopted a combined neurovascular team approach of surgical and endovascular treatment to deal with the challenges of these lesions—a treatment philosophy that we have previously described.3 The cerebrovascular surgeons and the neurointerventionists on the team discuss each patient and determine jointly the treatment strategy on a case-by-case basis. The Massachusetts General Hospital (MGH) grade is a comprehensive aneurysm grading system, previously described, that accurately predicts the risks of surgical aneurysm treatment based on the Hunt-Hess grade (1 point for Hunt-Hess grade 4–5), the Fisher Scale score (1 point for Fisher Scale score 3–4), patient age (1 point for age >50), aneurysm size (1 point for size >10 mm), and if the aneurysm is a giant posterior circulation lesion (1 point for posterior circulation aneurysm ≥25 mm).4 Good MGH grade patients tend to be treated with surgery, because of the higher rate of complete and durable occlusion, and poor MGH grade patients tend to be treated endovascularly.
Factors that are also important to treatment decision making are aneurysm size, neck size, aneurysm morphology, direction of the aneurysm, and aneurysm location in relation to the clivus (Table 20.1). Giant aneurysms (>25 mm) of the posterior circulation have been previously demonstrated by our group to be a higher surgical risk4; therefore, they tend to be treated by our group endovascularly rather than surgically. Wide neck sizes (>4 mm) tend to be unfavorable for endovascular treatment; therefore, our group has preferred surgical reconstruction for wide-necked aneurysms or aneurysms with an unfavorable dome-to-neck ratio. Similarly, aneurysm morphology is important as endovascular therapy is less favorable for aneurysms that incorporate either or both P1 arteries, in which case surgical reconstruction is performed. Drake5 defined the importance of aneurysm direction, and described forward-projecting aneurysm domes as tending to be free of perforators, whereas backward sacs are at risk of the clip blades closing on the perforators on the opposite side. Thus, we have tended to treat backward-projecting aneurysms endovascularly. Finally, we have previously described a classification system of basilar artery aneurysms based on the location of the aneurysm in relation to the clivus and the posterior clinoid.6 Aneurysms that are below the posterior clinoid and are low in relation to the top of the clivus are more challenging to approach surgically, and thus we have tended to treat these aneurysms endovascularly.
♦ Surgical Approaches
We have previously described surgical approaches to basilar artery aneurysms using a classification system based on the location of the aneurysm in relation to the clivus.6 High supraclival aneurysms are approached via a pterional or pterional with orbitozygomatic resection approach, supraclival aneurysms via a pterional or subtemporal or combined “half-and-half” approach, and upper clival aneurysms via a subtemporal transtentorial approach (Table 20.2).
Table 20.1 Factors for Deciding on Surgical or Endovascular Treatment for Basilar Apex Aneurysms
| Factor | Treatment Modality |
| MGH grade | |
| Good MGH grade (0–2) | Surgery > endovascular |
| Poor MGH grade (3–5) | Endovascular |
| Aneurysm size | |
| <15 mm | Surgery or endovascular |
| ≥15 mm | Endovascular |
| Aneurysm neck size | |
| <4 mm | Surgery or endovascular |
| ≥4 mm | Surgery |
| Aneurysm morphology | |
| Distinct from P1(s) | Surgery or endovascular |
| Incorporates P1(s) | Surgery |
| Aneurysm direction | |
| Projects forward | Surgery or endovascular |
| Projects backward | Endovascular |
| Aneurysm location to clivus | |
| High supraclival | Surgery or endovascular |
| Supraclival | Surgery or endovascular |
| Upper clival | Surgery or endovascular |
| Middle or low clival | Endovascular |
P1, first segment of the posterior cerebral artery (PCA).
The pterional approach was popularized by Yasargil et al7 and offers the advantage of less brain retraction than in the subtemporal approach. However, with the pterional approach, the line of sight is oblique, the working distance is longer, and low-lying lesions are obscured by the posterior clinoid process.6 The surgeon must work in a deep and narrow field in the opticocarotid triangle, or in a small space just lateral to the internal carotid artery.7 Adding orbitozygomatic resection to the pterional approach provides a view from the inferior direction with less retraction. We use a standard pterional approach used by many cerebrovascular surgeons.6 The patient is positioned supine with the head turned. We have preferred to use a lumbar cerebrospinal fluid drainage catheter. A standard pterional skin incision starts at the level of the zygoma just in front of the tragus and curves gently to the midline behind the hairline. The temporalis muscle is divided and flapped forward. A standard pterional craniotomy is performed, with further bony removal of the lateral sphenoid wing. The dura is opened in a curvilinear manner. The brain is relaxed by the administration of mannitol and by removal of cerebrospinal fluid from the lumbar drain. Further relaxation is achieved by suctioning of cerebrospinal fluid from above the optic nerve. The frontal lobe can then be gently and gradually retracted. The microscope is brought into view and the medial cisterns are then opened by arachnoid dissection. Two corridors for working are opened: the opticocarotid triangle, and a small space just lateral to the internal carotid artery. The membrane of Liliequist is opened, allowing access to the interpeduncular cistern. Isolation of the basilar artery is necessary for proximal vessel control. After temporary clip occlusion, dissection around the aneurysm can be performed, with utmost attention paid to brainstem and thalamic perforators. Final clip placement is performed with inspection of all sides of the aneurysm to ensure no perforating vessels are injured.
Table 20.2 Surgical Approaches to Basilar Apex Aneurysms Based on the Clival Location
| Clival Location | Surgical Approach |
| High supraclival | Pterional |
| Pterional with orbitozygomatic resection | |
| Supraclival | Pterional |
| Subtemporal | |
| Combined “half-and-half” | |
| Upper clival | Subtemporal transtentorial |
Drake5,8 popularized the subtemporal approach. The advantages are a short working distance and a perpendicular view of the aneurysm neck, but a disadvantage is the need for deep temporal retraction, particularly for high aneurysms.6 The approach is best for small basilar apex aneurysms or ones that project posteriorly. Our technique has been described previously.6 The patient is placed in the lateral position with the head elevated and the vertex dipped slightly to allow the temporal lobe to fall away from the floor of the middle fossa. A lumbar cerebrospinal fluid drainage catheter is used. We use a modified pterional type of skin incision, separate the temporalis muscle, and make a wide-based craniotomy with additional bone removed to maximize exposure of the floor of the middle fossa (Fig. 20.1). The dura is opened in a T- or I-fashion with the vertical limb extended down to the skull base. The temporal lobe is gently and gradually elevated and retracted, which is aided by temporal lobe relaxation from the administration of mannitol and removal of cerebrospinal fluid from the lumbar drain. The microscope is brought into view. The third cranial nerve is identified, and the approach to the aneurysm can then follow along a corridor inferior to the third nerve; however, we have found it helpful also to expose a corridor superior to the third nerve and use both corridors. The edge of the tentorium can be retracted laterally by tacking it up with sutures between the third and fourth cranial nerves. A wide arachnoid opening is performed between the third nerve and the superior cerebellar artery. Working along these corridors, dissection is performed to identify the basilar artery for proximal vessel control. Temporary clip occlusion of the basilar artery is performed before dissecting around the aneurysm. The utmost attention is paid to brainstem and thalamic perforators, with a view on all sides of the aneurysm necessary before and after final clip placement to avoid potential injury to these vessels.
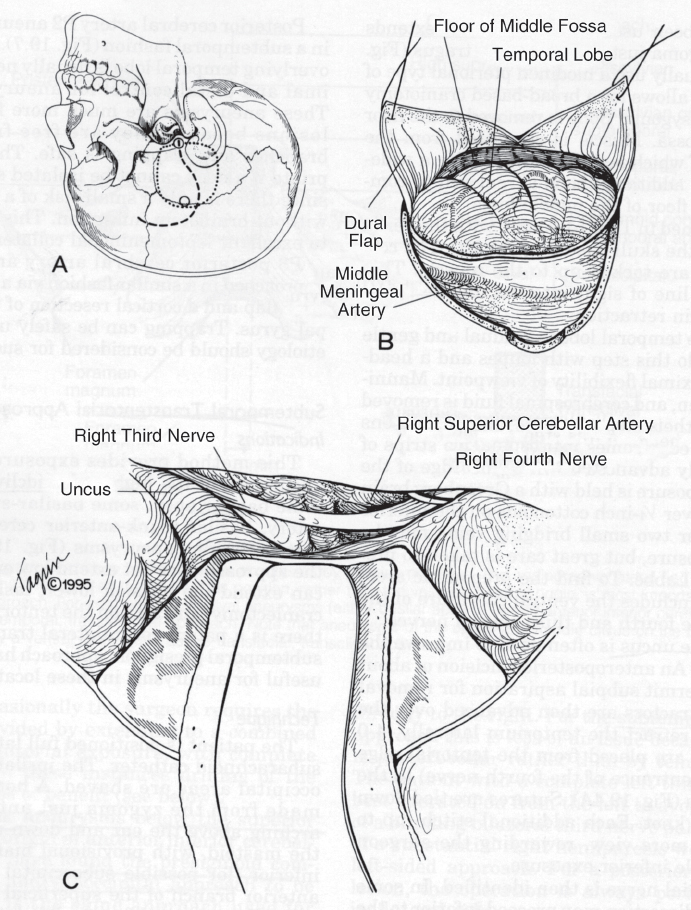

Fig. 20.1 Basic subtemporal approach. (A) Head position, incision, and craniotomy. (B) Dural opening. (C) Temporal lobe elevation. (D) Tentorial retraction and arachnoid opening. (E) Initial dissection of basilar artery. PICA, posterior inferior cerebellar artery. (From Ogilvy CS, Crowell RM, Heros RC. Basilar and posterior cerebral artery aneurysms. In: Ojemann RG, Ogilvy CS, Crowell RM, Heros RC, eds. Surgical Management of Neurovascular Disease, 3rd ed. Baltimore: Williams & Wilkins. 1995, reprinted by permission.)
The combined “half-and-half” approach had been first described by Drake9 and by Sano,10 who called it the “temporopolar approach,” but it is used by many cerebrovascular surgeons for basilar tip aneurysms. We use the technique described by Heros et al.6,11 The patient is positioned supine with the head turned to the opposite side. A lumbar cerebrospinal fluid drainage catheter is used. We use a modified pterional-type skin incision down to the zygoma, which facilitates a larger craniotomy (Fig. 20.2). The temporalis muscle is divided and retracted posteriorly, leaving a cuff of temporalis fascia attached to the periosteum to suture to at closure. A large wide-based craniotomy is made, and bone is removed down to the floor of the middle fossa, and the posterior pterion and greater sphenoid wing are removed to completely expose the dura over the anterior aspect of the temporal pole. The dura is opened. The pole of the temporal lobe is then gently and gradually retracted posteriorly, which is facilitated by temporal lobe relaxation by the administration of mannitol and removal of cerebrospinal fluid from the lumbar drain. The microscope is brought into view, at which point, the medial cisterns and sylvian fissure are opened by arachnoid dissection. The third cranial nerve is then identified, and two corridors are opened through which to work, medial and lateral to the nerve. The membrane of Liliequist is opened, providing access to the interpeduncular cistern. The basilar artery is identified for proximal vessel control. After temporary clip occlusion, dissection is performed around the aneurysm, and then final clip placement is performed.
The subtemporal transtentorial approach provides exposure for the upper clival laterally positioned basilar tip aneurysms. The technique we use has been described previously.6 The patient is placed in the full lateral position. A lumbar cerebrospinal fluid drainage catheter is used. The skin incision is in a horseshoe shape from the zygoma arching above the ear and down approximately 2 cm behind the mastoid, with the allowance for extending the incision 3 cm if a suboccipital craniectomy is later needed. A full wide temporal craniotomy is performed with additional bony removal as needed to expose the floor of the middle fossa. Openings into the mastoid air cells are waxed to prevent cerebrospinal fluid leakage. The dura is opened with an inferiorly based flap.
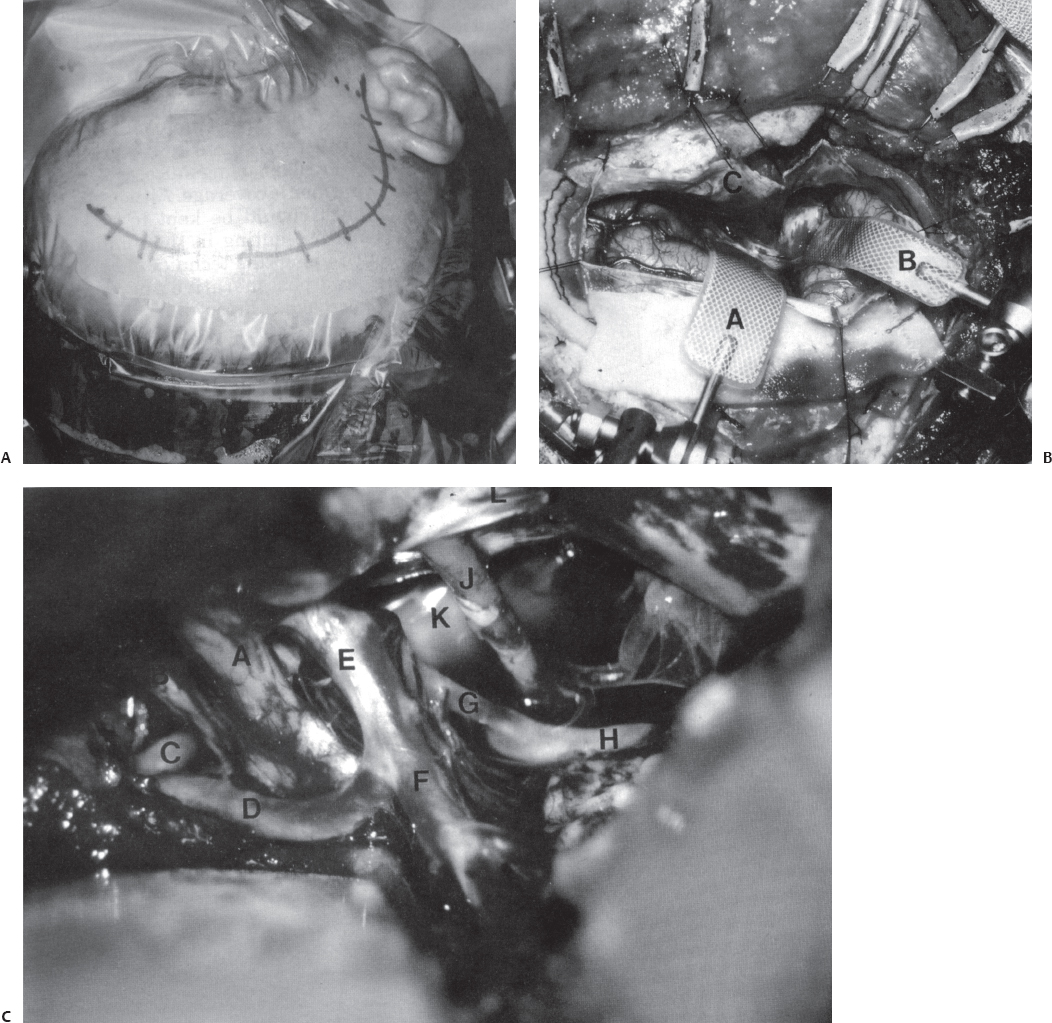
Fig. 20.2 Combined “half-and-half” approach. (A) Head position and skin incision. The dotted line indicates the level of the zygomatic arch. (B) The dura has been opened with a curvilinear inferior incision. A, retractor under the frontal lobe; B, retractor over the anterior temporal tip; C, inferior dural flap over the orbital roof. (C) Microsurgical exposure. The arachnoid has been opened widely and the sylvian fissure has been opened. A, right optic nerve; B, left optic nerve; C, left A1 artery; D, right A1 artery; E, right internal carotid artery; F, right middle cerebral artery; G, right posterior communicating artery; H, right posterior cerebral artery (P2); J, right ocular motor nerve; K, arachnoid over giant basilar artery; L, tentorial edge retracted with a suture. (From Ogilvy CS, Crowell RM, Heros RC. Basilar and posterior cerebral artery aneurysms. In: Ojemann RG, Ogilvy CS, Crowell RM, Heros RC, eds. Surgical Management of Neurovascular Disease, 3rd ed. Baltimore: Williams & Wilkins, 1995.)
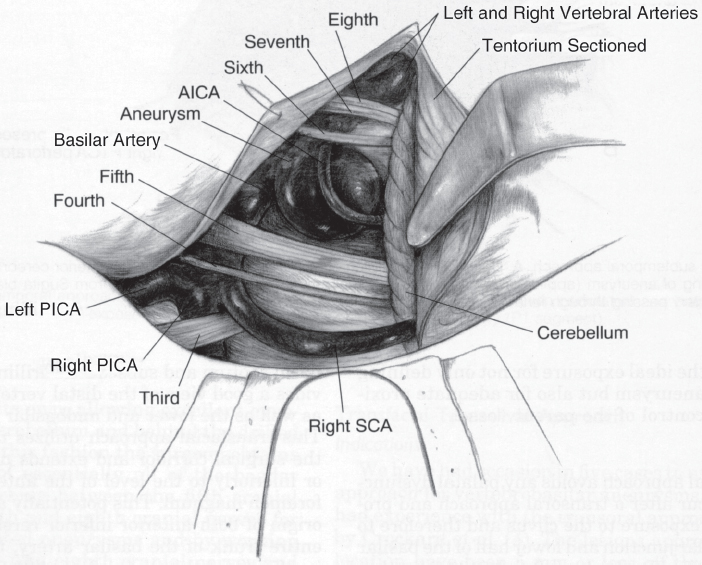
Fig. 20.3 Subtemporal transtentorial approach. Tentorium is sectioned behind the fourth cranial nerve 2 to 3 mm off the petrous ridge. A retractor on the cerebellum holds open the cerebellopontine angle. The line of sight is from the posterior direction. AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery. (From Ogilvy CS, Crowell RM, Heros RC. Basilar and posterior cerebral artery aneurysms. In: Ojemann RG, Ogilvy CS, Crowell RM, Heros RC, eds. Surgical Management of Neurovascular Disease, 3rd ed. Baltimore: Williams & Wilkins, 1995.)
The temporal lobe, which has been relaxed by the administration of mannitol and removal of cerebrospinal fluid from the lumbar drain, is gently and gradually elevated upward, as in the basic subtemporal approach; however, in this case, a more posterior edge of the tentorium is exposed. The microscope is brought into view and the fourth cranial nerve is identified. The tentorium is cut just behind the fourth cranial nerve and is then sectioned approximately 2 mm off the superior petrosal sinus and extending 3 to 4 cm posterolaterally, sparing the lateral sinus. This triangular flap is then excised, providing an excellent view of the clivus. Gentle retraction of the cerebellum exposes the cerebellopontine angle. The resulting view, which is best seen by angling the microscope from the posterior direction to see around the posterior clinoid process, is wide enough for temporary clip placement, aneurysm dissection, and final clipping (Fig. 20.3). In rare cases, a suboccipital craniectomy may be needed to further the exposure. The incision is extended 3 cm inferiorly, and a suboccipital craniectomy is performed over the sigmoid–transverse sinus junction. The tentorium is completely sectioned, including lateral sinus ligation (as long as the contralateral lateral sinus is patent), allowing for elevation of the entire temporo-occipital hemisphere, which produces maximal view onto the clivus.
♦ Results
From 1990 to 1998, the combined neurovascular team at the Massachusetts General Hospital treated 100 basilar tip aneurysms.3 Treatment was clipping in 72 patients and coiling in 28 patients. As mentioned above and in Table 20.1, our treatment strategy has been to treat good MGH grade patients with clipping and poor MGH grade patients with coiling. Aneurysm size, neck size, morphology, direction, and location in relation to the clivus also influence the selection of the treatment modality (Table 20.1). An angiographically complete occlusion was achieved with clipping in 60 of 64 patients with postoperative angiography (94%) (eight patients did not undergo postoperative angiography). Angiographic efficacy with coiling was 100% in eight patients (29%), ≥95% in eight patients (29%), and <95% in 12 patients (43%). Clinical outcomes as assessed by a slight modification of the Glasgow Outcome Scale (GOS)3 in the surgical group were excellent (GOS 5) in 46 patients (64%), good (GOS 4) in 14 patients (19%), fair (GOS 3) in six patients (8%), poor (GOS 2) in three patients (4%), and death (GOS 1) in three patients (4%). Clinical outcomes in the coiling group were excellent (GOS 5) in 17 patients (61%), good (GOS 4) in four patients (14%), fair (GOS 3) in four patients (14%), poor (GOS 2) in no patients, and death (GOS 1) in three patients (11%). There were no long-term posttreatment hemorrhages in the surgical group, but one coiled patient had a fatal posttreatment hemorrhage 5 years after initial coiling (the patients underwent a total of three coiling procedures for aneurysm recurrences).
♦ Conclusion
Basilar tip aneurysms have the worst natural history risk of all intracranial aneurysms1 and are associated with the highest treatment-related morbidity.1 They are challenging aneurysms for the cerebrovascular surgeon and the neurointerventionist. We have found that a combined neurovascular team approach offers the maximal chance for achieving the best clinical outcome and angiographic result while minimizing the risks of treatment-related morbidity.
References
1. Wiebers DO, Whisnant JP, Huston J III, et al; International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103–110 PubMed
2. Lawton MT. Basilar apex aneurysms: surgical results and perspectives from an initial experience. Neurosurgery 2002;50:1–8, discussion 8–10 PubMed
3. Ogilvy CS, Hoh BL, Singer RJ, Putman CM. Clinical and radiographic outcome in the management of posterior circulation aneurysms by use of direct surgical or endovascular techniques. Neurosurgery 2002;51:14– 21, discussion 21–22 PubMed
4. Ogilvy CS, Carter BS. A proposed comprehensive grading system to predict outcome for surgical management of intracranial aneurysms. Neurosurgery 1998;42:959–968, discussion 968–970 PubMed
5. Drake CG. Basilar artery bifurcation aneurysms. In: MLJ A, ed. Brain Surgery: Complication Avoidance and Management, 1st ed. New York: Churchill Livingstone, 1993:1041–1048
6. Ogilvy CS, Crowell RM, Heros RC. Basilar and posterior cerebral artery aneurysms. In: Ojemann RG, Ogilvy CS, Crowell RM, Heros RC, eds. Surgical Management of Neurovascular Disease, 3rd ed. Baltimore: Williams & Wilkins, 1995:269–290
7. Yasargil MG, Antic J, Laciga R, Jain KK, Hodosh RM, Smith RD. Microsurgical pterional approach to aneurysms of the basilar bifurcation. Surg Neurol 1976;6:83–91 PubMed
8. Drake CG. Bleeding aneurysms of the basilar artery. Direct surgical management in four cases. J Neurosurg 1961;18:230–238 PubMed
9. Drake CG. Comment on: Samson DS, Hodosh RM, Clark WK. Microsurgical evaluation of pterional approach to aneurysms of the distal basilar circulation. Neurosurgery 1978;3:140–141
10. Sano K. Temporo-polar approach to aneurysms of the basilar artery at and around the distal bifurcation: technical note. Neurol Res 1980;2: 361–367 PubMed
11. Heros RC, Lee SH. The combined pterional/anterior temporal approach for aneurysms of the upper basilar complex: technical report. Neurosurgery 1993;33:244–250, discussion 250–251 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








