Bell Palsy and Cranial Neuropathies
Comana M. Cioroiu
Thomas H. Brannagan III
Isolated cranial neuropathies are not uncommon, the most frequently encountered of which is Bell palsy. These syndromes can be seen in both the inpatient and outpatient settings, and all have a very varied differential diagnosis. The cranial nerve examination is a crucial component of a complete neurologic exam. Cranial nerve injury can be implicated in various diseases with widespread neurologic involvement such as stroke, multiple sclerosis, and demyelinating neuropathies, particularly when other pathways and neuroanatomic regions are involved as well. Cranial neuropathies can occur together (with involvement of more than one nerve) or in isolation (with involvement of only one nerve). Multiple cranial neuropathies are most often caused by cancer, infarct, and trauma, and these etiologies must be considered and carefully excluded when evaluating these patients, with particular attention paid to the brain stem where several cranial nerves localize together. However, occasionally, there may be abnormal findings limited to one cranial nerve in isolation. In these instances of isolated cranial neuropathies, the differential diagnosis depends on the nerve involved and the clinical picture as a whole. This chapter will address common cranial mononeuropathies and their evaluation and management. Several of these are addressed in other chapters of this text and are referred to when appropriate.
THE OLFACTORY NERVE (CRANIAL NERVE I)
The ability to smell is a special quality relegated to the olfactory cells in the nasal mucosa. The molecular biology of smell is uncertain, but transcription-activating factors, such as Olf-1, found exclusively in neurons with olfactory receptors, probably direct cellular differentiation. Smell may be impaired after injury of the nasal mucosa, the olfactory bulb or its filaments, or central nervous system (CNS) connections. Nerve injury causes diminution or loss of the sense of smell. The most common complaint of patients with olfactory nerve injury, however, is not loss of smell but diminished taste; olfaction plays a key role in taste perception because of the volatile substances in many food and beverages. A loss of sense of smell may be congenital or acquired and occurs in various conditions (Table 86.1). The sense of smell is most commonly impaired, transiently, because of allergic nasal congestion or the common cold. The most common traumatic olfactory nerve injury occurs in head injury, usually of the acceleration-deceleration variety, including motor vehicle accidents. The delicate olfactory nerve filaments are sheared by the perforations of the cribriform plate. The olfactory bulb can also be contused or lacerated in head injuries. Leigh and Zee (2006) reported altered olfactory sense in 7.2% of patients with head injuries at a military hospital, with complete loss in 4.1% and partial loss in 3.1%. Recovery of smell occurred in only 6 of 72 patients. In a study of head injuries in civilians, Friedman and Merritt (1944) found that the olfactory nerve was damaged in 11 (2.6%) of 430 patients. In all patients, anosmia was bilateral. In three, the loss was transient and disappeared within 2 weeks of injury. Parosmia (i.e., perversion of sense of smell) was present in 12 patients.
Inflammatory or neuritic lesions of the bulb or tract are uncommon, but these structures are sometimes affected in meningitis or in mononeuritis multiplex. Rarely, patients with diabetes mellitus have impaired smell, sometimes stemming from olfactory nerve infarction. Hyposmia or anosmia is also common early in Refsum disease (an autosomal recessive disease leading to an overaccumulation of phytanic acid). Kallmann syndrome is an X-linked inherited disorder causing hypogonadism and anosmia due to olfactory tract hypoplasia. The olfactory bulb or tract may be compressed by meningiomas (particularly at the olfactory groove or sphenoidal ridge), metastatic tumors, or aneurysms in the anterior fossa or by infiltrating tumors of the frontal lobe. The Foster-Kennedy syndrome is a classic syndrome caused by a tumor invading the orbitofrontal region, leading to unilateral and ipsilateral optic atrophy, contralateral papilledema, and anosmia. Neurodegenerative diseases at times may be heralded by a loss of smell, and this is seen particularly with Parkinson disease, where loss of smell may be a presenting sign. Certain drugs are often implicated in a loss of smell, particularly cocaine when used intranasally, although other toxins such as cadmium and chemotherapeutic agents have been implicated as well.
Parosmia is not accompanied by impairment of olfactory acuity and is most commonly caused by lesions of the temporal lobe, although it has been reported with injury to the olfactory bulb or tract.
Olfactory hallucinations may occur in psychosis or as a seizure aura that involves the hippocampal or uncinate gyrus; perceptions are described as strange, unpleasant, and ill-defined odors. Increased sensitivity to olfactory stimuli is generally rare, although it may occur in migraineurs and in patients with reactive airways disease, perhaps because of prior sensitization to olfactory triggers. Cases in which the sense of smell is so acute that it is a source of continuous discomfort, however, may be psychogenic.
Olfactory hallucinations may occur in psychosis or as a seizure aura that involves the hippocampal or uncinate gyrus; perceptions are described as strange, unpleasant, and ill-defined odors. Increased sensitivity to olfactory stimuli is generally rare, although it may occur in migraineurs and in patients with reactive airways disease, perhaps because of prior sensitization to olfactory triggers. Cases in which the sense of smell is so acute that it is a source of continuous discomfort, however, may be psychogenic.
TABLE 86.1 Causes of Loss of Smell Related to Olfactory Nerve Injury | ||||
|---|---|---|---|---|
|
THE OPTIC NERVE (CRANIAL NERVE II) AND CRANIAL NERVES III, IV, AND VI
Disorders of the visual system are described in Chapter 9.
THE TRIGEMINAL NERVE (CRANIAL NERVE V)
The fifth cranial nerve, or the trigeminal nerve, has both a large sensory component as well as a smaller motor component. The nerve has three major branches—the ophthalmic division (V1), the maxillary division (V2), and the mandibular division (V3). These three branches carry sensory information from distinct dermatomes of the face, head, and mucous membranes and converge on the trigeminal or gasserian ganglion located in Meckel’s cave (which serves as the dorsal root ganglion). Sensory fibers from all three divisions ascend in the pons to terminate in the three parts of the trigeminal nucleus. The spinal trigeminal nucleus receives afferent fibers related to facial pain and temperature sensation, the principal sensory nucleus receives fibers related to light touch and mechanoreception, whereas the mesencephalic nucleus contains cell bodies with fibers carrying information regarding jaw proprioception. Motor branches originate in the motor nucleus of the trigeminal nerve and are distributed in the mandibular division to innervate the muscles of mastication.
Injury to the fifth cranial nerve causes loss of soft-tactile, thermal, and pain sensation in the face; loss of the corneal and sneezing (i.e., sternutatory) reflexes; and paralysis of the muscles of mastication. Lesions of the trigeminal pathways in the pons usually affect the motor and chief sensory nuclei causing paralysis of the muscles of mastication and loss of light touch perception in the face; lesions in the medulla affect only the descending tract and cause loss of facial light touch sensation. Brain magnetic resonance imaging (MRI) with contrast is often useful to search for mass lesions, ischemia, and inflammation, whereas electrophysiologic testing (e.g., blink reflex testing) may help quantitate both the afferent (i.e., trigeminal nerve) and efferent (i.e., facial nerve) components of the corneal reflex. The fifth nerve may be injured by trauma, neoplasm, aneurysm, or meningeal infection. Infarcts and other vascular lesions, as well as intramedullary tumors, may damage the sensory and motor nuclei in the pons and medulla. Isolated lesions of the descending tract may occur in syringobulbia or in multiple sclerosis. Common causes of trigeminal nerve injury with facial numbness include dental or cranial trauma, herpes zoster, head and neck tumors, intracranial tumors, and idiopathic trigeminal neuropathy. Less common causes include multiple sclerosis, systemic sclerosis, mixed connective tissue diseases, amyloidosis, and sarcoidosis. Isolated facial numbness may also occur without a clearly identifiable cause (i.e., idiopathic trigeminal neuropathy), but these patients must be carefully evaluated to ensure an occult process is not overlooked. Although restricted loss of sensation over the chin (i.e., the numb chin syndrome) usually is caused by dental trauma, dental or surgical procedures, or even poorly fitting dentures, this syndrome is a recognized initial feature of a systemic malignancy such as lymphoma, metastatic breast carcinoma, melanoma, or prostate cancer. MRI of the mandible may help separate these disorders. Painful facial numbness may herald nasopharyngeal or metastatic carcinoma. Isolated weakness of the trigeminal innervated muscles of mastication may be seen in motor neuron disease where patients often develop jaw weakness and dysphagia. This can also be seen with diseases of the neuromuscular junction, that is, myasthenia gravis.
TRIGEMINAL NEURALGIA
Epidemiology and Pathobiology
Trigeminal neuralgia, also commonly known as tic douloureux, is a syndrome of extremely severe facial pain without numbness or objective findings in the fifth nerve distribution (see also Chapter 55). This disorder of the trigeminal nerve is characterized by recurrent paroxysms of sharp, stabbing pains in the distribution of one or more nerve branches. Unlike herpes zoster, the second and third divisions of the trigeminal nerve are the most commonly involved and the first is primarily affected in only less than 5% of patients. Onset is usually in middle or late life but may occur at any age. Typical trigeminal neuralgia occasionally affects children but rarely occurs before age 35 years—presentation at that age should prompt an investigation for demyelinating disease. The incidence of trigeminal neuralgia is slightly greater in women than in men and found to be about 12.6 per 100,000 people in some studies.
The cause remains unknown. In most cases, no organic disease of the fifth nerve or the CNS is identified. Degenerative or fibrotic changes in the gasserian ganglion have been described but are too variable to be considered causal. Trigeminal nerve compression related to an anomalous blood vessel, usually in the vicinity of the ganglion, is a long-standing but controversial etiology of the disorder. Most commonly, this is thought to be compression by a loop of either the anterior inferior cerebellar or superior cerebellar artery. Painful symptoms typical of trigeminal neuralgia occasionally occur with demyelinating brain stem lesions including those produced by multiple sclerosis, as well as vascular ischemia affecting the descending root of the fifth nerve. When trigeminal neuralgia has a known structural cause, it is categorized as symptomatic, as opposed to the idiopathic form that has no known etiology. Although trigeminal neuralgia usually follows other symptoms of multiple sclerosis rather than precedes them, up to 10% of patients may have facial pain as part of their initial presentation. Tumors invading the gasserian ganglion or the cerebellopontine angle may also cause symptoms of trigeminal neuralgia, although usually in the setting of an abnormal neurologic examination. The paroxysmal attacks of facial pain in trigeminal neuralgia may be related to excessive discharge within the descending nucleus of the nerve triggered by an influx of impulses. Relief of symptoms by section of the greater auricular or occipital nerves in some patients suggests a role for peripheral excitation, and interruption of an episode by intravenous phenytoin, as well as a general therapeutic response to antiepileptic agents, suggests aberrant neuronal discharge may also play an important part in the pathophysiology of this disorder. Trigeminal neuralgia is the most common of all neuralgias.
Clinical Manifestations and Diagnosis
The pain is extremely severe, is described by many patients as among the worst pain imaginable, and in severe and refractory cases,
the risk of suicide is increased. The pain appears in paroxysms and typically lasts seconds, although episodes of up to 15 minutes can occur. Between episodes, the patient is free of symptoms, except for fear of an impending attack. The pain is searing or burning, coming in lightning-like jabs. The frequency of attacks varies from many times a day to a few times a month. The patient ceases to talk when the pain strikes and may rub or pinch the face; movements of the face and jaw may accompany the pain. Sometimes, ipsilateral lacrimation is prominent. No objective loss of cutaneous sensation is found during or after the paroxysms, but the patient may complain of facial hyperesthesia.
the risk of suicide is increased. The pain appears in paroxysms and typically lasts seconds, although episodes of up to 15 minutes can occur. Between episodes, the patient is free of symptoms, except for fear of an impending attack. The pain is searing or burning, coming in lightning-like jabs. The frequency of attacks varies from many times a day to a few times a month. The patient ceases to talk when the pain strikes and may rub or pinch the face; movements of the face and jaw may accompany the pain. Sometimes, ipsilateral lacrimation is prominent. No objective loss of cutaneous sensation is found during or after the paroxysms, but the patient may complain of facial hyperesthesia.
A characteristic feature in the presentation is the trigger zone, stimulation of which sets off a typical paroxysm of pain. This zone is a small area on the cheek, lip, or nose that may be stimulated by facial movement, chewing, brushing teeth, or touch. The patient may avoid making facial expressions during conversation, may go without eating for days, or may avoid the slightest breeze to prevent an attack. The pain is limited strictly to one or more branches of the fifth nerve and does not spread beyond the distribution of that nerve. The second division is involved more frequently than the third. Pain may spread to one or both of the other divisions. In cases of long duration, all three divisions are affected in 15% of patients. The pain is occasionally bilateral (5%) for some but rarely occurs at the same time. Bilateral trigeminal neuralgia is encountered most often in patients with multiple sclerosis. A new classification scheme differentiates classic trigeminal neuralgia with paroxysms of pain from a different form, in which the paroxysms are associated with a concomitant dull, constant facial pain.
The diagnosis of trigeminal neuralgia is usually made from the history. Neurologic examination in patients with trigeminal neuralgia is usually normal, although some patients may also have concurrent hemifacial spasm, and patients whose attacks are provoked by eating may appear thin or cachectic. The results of serum studies and other diagnostic evaluations are also normal. Characteristically, patients avoid touching the area of origin when asked to point it out, instead holding the tip of the index finger a short distance from the face. On examination, patients will show no clinical sensory or motor deficits in the trigeminal nerve distribution, although the affected area is often very sensitive. Computed tomography (CT) or MRI of the brain is reasonable to exclude structural causes and at times may demonstrate an aberrant vessel causing compression. However, the sensitivity and specificity of MRI for identifying neurovascular compression is variable and thus its role for this purpose is controversial. In 2008, the American Academy of Neurology put forth a practice parameter concerning the diagnosis and treatment of trigeminal neuralgia, which stated that electrodiagnostic evaluation of the trigeminal reflex is a reasonable first step in excluding symptomatic trigeminal neuralgia and can be considered before imaging.
Trigeminal neuralgia must be differentiated from other types of facial pain or headache, especially infections of the teeth and nasal sinuses. These pains are usually steady instead of episodic, are often throbbing, and persist for many hours. However, it is not uncommon for patients with trigeminal neuralgia to undergo surgical treatment of the sinuses and/or tooth extractions before the diagnosis is established. Conversely, patients with diseased teeth may be referred to neurology with a diagnosis of trigeminal neuralgia, although careful dental examination usually identifies the teeth as the source of pain in these patients.
Temporomandibular joint disease may also mimic trigeminal neuralgia, but the pain is not paroxysmal and, although exacerbated by eating, no trigger point may be identified and symptoms are usually less severe between meals. Cluster headaches are another consideration but occur in protracted clusters rather than as brief events and are accompanied by ipsilateral nasal congestion, ipsilateral conjunctival injection and lacrimation, and an ipsilateral Horner syndrome. Atypical facial pain may have a trigeminal distribution but the individual paroxysms always last longer than a few seconds (usually minutes or hours). The pain itself is dull, aching, crushing, or burning. Surgical treatment is not effective in atypical facial pain and its etiology remains obscure, although it may be associated with depression.
A more detailed discussion of headache and facial pain syndromes can be found in Chapter 7.
Treatment and Outcome
Although surgical options now exist, medical therapy remains first line as far as treatment options. Trigeminal neuralgia is most effectively treated with carbamazepine at 800 mg/day to a maximum dose of 1,500 mg/day in four divided doses. A Cochrane Database review found carbamazepine to be consistently effective, with a number needed to treat of 1.8. However, dosing should be titrated to effect, and doses producing serum levels above the therapeutic range for seizure control may be needed as long as potentially dose-limiting side effects are tolerated. Overdosage is manifested by drowsiness, dizziness, ataxia, unsteady gait, and nausea. Hepatotoxicity may occur but is usually reversible with discontinuation. Another more rare but serious complication is aplastic anemia, and both periodic liver function testing and monitoring of the blood count are needed. In some patients, tolerance may develop over time. Baclofen is also effective in many cases in doses of 40 to 80 mg/day; phenytoin is less effective but may be used as adjunctive therapy. Some of the newer antiepileptic agents may also provide some relief, and oxcarbazepine, a derivative of carbamazepine, in doses of 400 to 1,200 mg/day is thought to be as effective as carbamazepine. Other medications which have been shown to be effective in some cases (although no controlled trials exist) include lamotrigine, gabapentin, pregabalin, sumatriptan, and topiramate. More recently, both intravenous lidocaine and onabotulism toxin A have been found to be successful in treating acute episodes of trigeminal neuralgia, although more rigorous trials are needed. Surgical procedures used to treat this condition include microvascular decompression, radiofrequency ablation, balloon microcompression, and chemical gangliolysis and rhizotomy. More recently, stereotactic radiosurgery using Gamma Knife has been used with noted improvements in pain scores. Of these methods, radiofrequency ablation has met with the greatest success in initial treatment, although recurrence rates have not been studied carefully. As compression of the trigeminal nerve by arterial loops may play a role in some cases, posterior fossa exploration with decompression has sometimes been used for refractory cases. Other chronic masses, such as arteriovenous malformation, aneurysm, and cholesteatoma, may also cause compression of the ganglion and may be more amenable to surgical correction.
THE FACIAL NERVE (CRANIAL NERVE VII)
The seventh cranial nerve (facial nerve), although predominantly motor, also serves an important parasympathetic and sensory function (Fig. 86.1). On exiting the brain stem ventrally via the internal acoustic meatus in the petrous part of the temporal bone near the pontomedullary junction, the facial nerve forms two divisions: the nervus intermedius and the motor root. The motor root is
composed of nerve fibers arising from the facial motor nucleus, and after exiting the nucleus, these axons travel dorsomedially in the pons to circle the abducens nucleus (thus forming the facial colliculus) and travel down to meet the nervus intermedius to form the facial nerve. The nervus intermedius arises from superior salivatory and lacrimal nuclei (eventually sending parasympathetic innervation to the salivary glands, specifically the submaxillary and sphenopalatine ganglia), the nucleus solitarius (fibers of which ultimately relays afferent taste sensation from the anterior two-thirds of the tongue), and the spinal trigeminal nucleus (carrying somatosensory afferent fibers to parts of the face and ear). The fibers of the seventh cranial nerve arising from the spinal trigeminal nucleus may also relay proprioceptive impulses from the facial muscles and cutaneous sensation from the posteromedial surface of the pinna and the external auditory canal. The fibers arising from the nucleus solitarius and spinal trigeminal nucleus together synapse in the geniculate ganglion in close proximity to the brain stem. Distal to the geniculate ganglion, the facial nerve forms several branches. Axons from the superior lacrimal nucleus form the greater petrosal nerve, which synapses in the sphenopalatine ganglion prior to innervating the lacrimal glands. Fibers originating from the nucleus solitarius and the superior salivatory and nuclei travel further distally in the facial nerve prior to forming the chorda tympani, a branch of the nerve which crosses the middle ear and exits the skull to join the lingual nerve. Just prior to the chorda tympani, the facial nerve also gives off a motor branch to the stapedius muscle. Distal to the chorda tympani, the motor root of the facial nerve runs through the facial canal and exits the skull via the stylomastoid foramen. At this point, it gives rise to the posterior auricular nerve innervating the scalp and ear, as well as a motor branch to the stylohyoid muscle and digastric muscle. The nerve then continues on into the parotid gland, where it divides into its five major branches—temporal, zygomatic, buccal, marginal mandibular, and cervical.
composed of nerve fibers arising from the facial motor nucleus, and after exiting the nucleus, these axons travel dorsomedially in the pons to circle the abducens nucleus (thus forming the facial colliculus) and travel down to meet the nervus intermedius to form the facial nerve. The nervus intermedius arises from superior salivatory and lacrimal nuclei (eventually sending parasympathetic innervation to the salivary glands, specifically the submaxillary and sphenopalatine ganglia), the nucleus solitarius (fibers of which ultimately relays afferent taste sensation from the anterior two-thirds of the tongue), and the spinal trigeminal nucleus (carrying somatosensory afferent fibers to parts of the face and ear). The fibers of the seventh cranial nerve arising from the spinal trigeminal nucleus may also relay proprioceptive impulses from the facial muscles and cutaneous sensation from the posteromedial surface of the pinna and the external auditory canal. The fibers arising from the nucleus solitarius and spinal trigeminal nucleus together synapse in the geniculate ganglion in close proximity to the brain stem. Distal to the geniculate ganglion, the facial nerve forms several branches. Axons from the superior lacrimal nucleus form the greater petrosal nerve, which synapses in the sphenopalatine ganglion prior to innervating the lacrimal glands. Fibers originating from the nucleus solitarius and the superior salivatory and nuclei travel further distally in the facial nerve prior to forming the chorda tympani, a branch of the nerve which crosses the middle ear and exits the skull to join the lingual nerve. Just prior to the chorda tympani, the facial nerve also gives off a motor branch to the stapedius muscle. Distal to the chorda tympani, the motor root of the facial nerve runs through the facial canal and exits the skull via the stylomastoid foramen. At this point, it gives rise to the posterior auricular nerve innervating the scalp and ear, as well as a motor branch to the stylohyoid muscle and digastric muscle. The nerve then continues on into the parotid gland, where it divides into its five major branches—temporal, zygomatic, buccal, marginal mandibular, and cervical.
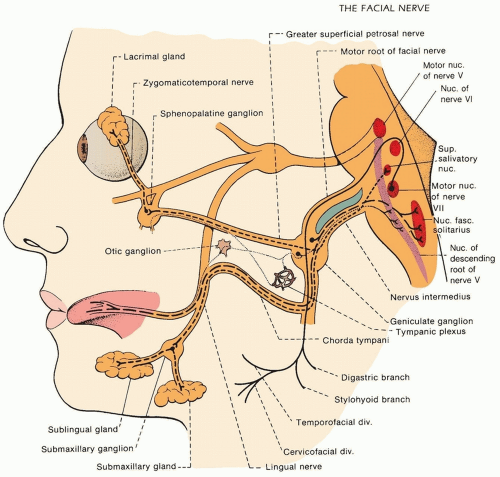 FIGURE 86.1 Anatomy of the facial nerve. (From Campbell WW. DeJong’s The Neurologic Examination. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2005.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





