BRAIN ABSCESS
A brain abscess is a focal purulent infection within the brain parenchyma. A brain abscess starts as a localized area of cerebritis; abscesses vary in size from a microscopic collection of inflammatory cells to an area of purulent necrosis presenting as a mass lesion.
Advances in the diagnosis and treatment of brain abscesses have been achieved with the use of computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), stereotactic brain biopsy and aspiration, and newer broad-spectrum antibiotics.
EPIDEMIOLOGY
A brain abscess is two to three times more common in males than in females, with the highest morbidity in fourth decade of life. Over the past 30 years, advances in management of brain abscesses have resulted in a significant decline in mortality from 30% to 50% before 1980, to 4% to 20% today. Up to 25% of all cases occur in children younger than 15 years old, with a cluster in the 4- to 7-year-old age group, usually the result of cyanotic congenital heart disease or an otic infection.
ETIOLOGY
A brain abscess is classified based on the likely source of infection. A brain abscess may develop from any of the following predisposing or associated conditions: (1) direct extension of cranial infection, ethmoid or frontal sinusitis, subacute or chronic otitis media, or mastoiditis; (2) hematogenous dissemination of infection from elsewhere in the body (e.g., lung abscess, bacterial endocarditis, intra-abdominal infections, dental infections, congenital heart disease); and (3) direct inoculation via wounds, fracture of the skull, or neurosurgical procedures.
Infection in the middle ear or mastoid may spread to the cerebellum or temporal lobe through involvement of bone and meninges or by seeding of bacteria through valveless emissary and sinus veins that drain these regions. Infection may originate from tonsils or abscessed teeth, or frontal, ethmoid, or rarely the maxillary sinuses, with sinus infections often spreading to frontal lobes through erosion of the skull.
For hematogenous spread, cerebral lesions are found in distal territories of the middle cerebral artery and frequently are multiple, often seen at the junction of gray-white matter. Potential sources for hematogenous spread of infection include upper respiratory tract (lung abscess or empyema), bacterial endocarditis, skin infections, intra-abdominal, or pelvic infections. Twenty percent to 30% of brain abscesses have no obvious source, and are so-called cryptogenic brain abscesses. In some cases, cryptogenic brain abscess may have a cardiac or lung source. For example, congenital cardiac defects and pulmonary arteriovenous malformations (e.g., hereditary hemorrhagic telangiectasia) predispose to brain abscess. In these two disorders, infected emboli bypass pulmonary filtration system and gain access to the cerebral arterial system. Similarly, cases of cryptogenic brain abscess caused by vegetations on patent foramen ovale (PFO) or right-to-left shunting via PFO have been reported. In infants and children, congenital heart disease is a common predisposing factor for brain abscess. The predisposing conditions of hereditary hemorrhagic telangiectasia or Rendu-Osler-Weber disease can be the source of a brain abscess via paradoxical septic emboli from lung.
A brain abscess can occur following penetrating head injury or neurosurgical procedures, although occurrence of abscesses after penetrating brain injury is low. In children, penetration of a lead pencil tip through the thin squamous portion of the temporal bone has resulted in abscess formation. Finally, brain abscess is almost never a consequence of bacterial meningitis, except in infants.
CAUSATIVE ORGANISMS OF BRAIN ABSCESS
The infecting organism may be any of the common pyogenic bacteria depending on the site of entry; the most common are
Staphylococcus aureus, streptococci (anaerobic, aerobic, or microaerophilic species),
Enterobacteriaceae,
Pseudomonas spp., and anaerobes such as
Bacteroides spp. (
Table 62.1). In infants, gramnegative organisms are the most frequent isolates. In adult patients with direct spread of infection from an otogenic source, the most common organisms are streptococci,
Bacteroides spp.,
Pseudomonas spp.,
Enterobacteriaceae, and
Haemophilus. Other causative organisms from oral or hematogenous spread include streptococci,
S. aureus,
Bacteroides spp., and
Fusobacterium spp. After penetrating head injury or neurosurgical procedure, abscess formation is usually due to
S. aureus (methicillin-resistant
Staphylococcus aureus or MRSA),
Staphylococcus epidermidis, streptococci,
Enterobacteriaceae,
Pseudomonas, or
Clostridium spp. Brain abscesses as a complication of bacterial endocarditis are due to streptococci, MRSA, and
Serratia marcescens. In the immunocompromised host,
Toxoplasma,
Listeria, Nocardia, Aspergillus, Cryptococcus, Coccidioidomycosis, Mycobacterium tuberculosis, Enterobacteriaceae, and other fungal pathogens are causative organisms of brain abscess. Brain abscess may be due to a parasitic infection such as
Entamoeba histolytica, cysticercosis,
Schistosoma, or toxocariasis. Following liver transplantation, invasive pulmonary aspergillosis can be a source of brain abscesses. Cultures may be sterile in patients who have received antimicrobial therapy before biopsy but any material obtained should be sent to the laboratory. A positive Gram stain guides therapy even when the culture is negative. In a study of 90 patients with diagnosis of brain abscess, microbiologic diagnosis was obtained in 83% of cases. More than one pathogen was found in 23% of cases.
PATHOBIOLOGY
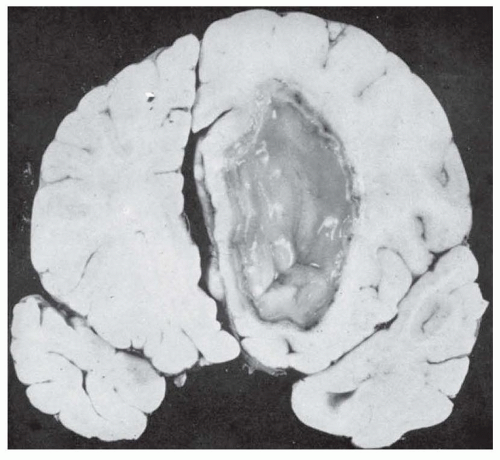
The pathologic changes in brain abscesses are similar regardless of the origin: direct extension to the brain from a contiguous site of infection, retrograde thrombosis of veins, or hematogenous dissemination (
Fig. 62.1).
Four stages of maturation of brain abscess are recognized: (1) within the first 3 days, suppurative inflammation of brain tissue is characterized by early cerebritis, appearing as either a patchy or nonenhancing hypodensity on CT or MRI; (2) late cerebritis (days 4 to 9) with an area of central necrosis, edema, and ring enhancement on CT and MRI; (3) early capsule formation (days 10 to 14); and (4) final maturation of the capsule, which takes about 2 weeks. When host defenses control the spread of the infection, macroglia and fibroblasts proliferate in an attempt to surround the infected and necrotic tissue, and granulation tissue and fibrous encapsulation develop. The developing capsule around the abscess formed by the inflammatory response is thicker on the cortical surface than on the ventricular side. If the capsule ruptures, purulent material is released into the ventricular system, a process associated with a high mortality rate. Edema surrounding a brain abscess is common.
Two signal-mediated CNS immune responses to invading pathogens that may be important in the developing abscess include the Toll-like and Nod-like receptors (TLR and NLR, respectively) systems. Activation of TLR by invading pathogens leads to a complex array of immune-mediated response including control of infection burden, immune infiltrates, and inflammatory mediators. Likewise, NLR activates a number of immune-specific interleukins responsible for bacterial recognition and subsequent cytokine release that acts to restrict bacterial spread during abscess formation.
CLINICAL MANIFESTATIONS
The symptoms of a brain abscess are typical of an expanding mass lesion in the brain with elevated intracranial pressure (ICP) and include headache with or without fever as the initial symptoms, followed by nausea, vomiting, lethargy, and focal neurologic deficits. Fever is present in approximately 50% of patients; however, the combination of fever and leukocytosis is present in over 50% cases of brain abscess. Sudden worsening of preexisting headache, newonset nuchal rigidity, and seizures may herald rupture of brain
abscess into the ventricular space. The abrupt onset of a severe headache is less common with abscess and is more often associated with acute bacterial meningitis or subarachnoid hemorrhage. Seizures, focal or generalized, are common with abscess.
Focal neurologic deficits develop in approximately 50% of patients, depending on abscess location. Hemiparesis, apathy, and confusion may be seen with lesions of the frontal lobe, hemianopia, and aphasia, particularly anomia, with abscesses in the temporal or parietooccipital lobes and ataxia, intention tremor, nystagmus, with cerebellar abscess (
Fig. 62.2). The presence of papilledema and/or third or sixth cranial nerve palsies may be due to increased ICP.
Abscesses in the brain stem are rare. The classic findings of brain stem syndromes are often lacking because abscess tends to expand longitudinally along fiber tracts rather than transversely. Subdural or, rarely, epidural infections in the frontal regions may present with the same signs and symptoms as those of an abscess in the frontal lobe. Fever and focal seizures favor the diagnosis of subdural rather than intraparenchymal abscess, although these findings are not specific.
Transverse sinus thrombosis often follows middle ear or mastoid infection (see
Fig. 62.2) and may be accompanied by seizures and signs of increased ICP, making the clinical differentiation between this condition and abscess of the temporal lobe or hemispheres difficult. With transverse sinus thrombosis, papilledema may result from blockage of venous drainage from the brain. Focal neurologic signs favor the diagnosis of abscess.
DIAGNOSTIC TESTING
Brain abscess should be suspected when seizures, focal neurologic signs, or increased ICP develop in a patient with known acute or chronic infection in the middle ear, mastoid, nasal sinuses, heart, or lungs and in those with congenital heart disease. Neuroimaging by CT or MRI is essential to making the diagnosis.
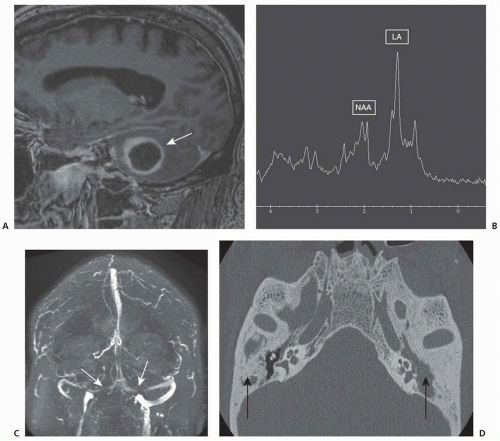
Although CT scan can be useful, MRI neuroimaging is the neuroimaging study of choice, both for diagnosis and treatment of brain abscess. MRI with gadolinium is more sensitive and specific than contrast CT in diagnosing early cerebritis. On neuroimaging, abscesses are frequently found in the gray-white junction in watershed regions between vascular territories. Serial MRI permits accurate localization of cerebritis or abscess and serial assessment of the size of the lesion, its demarcation, the extent of surrounding edema, and total mass effect and allows for staging of the abscess. MRS may help differentiate brain abscesses based on metabolic patterns of lactate, amino acids, or acetate from other similar appearing lesions (e.g., tumor). The presence of lactate, amino acids, acetate, and succinate on MRS is considered specific for brain abscess (see
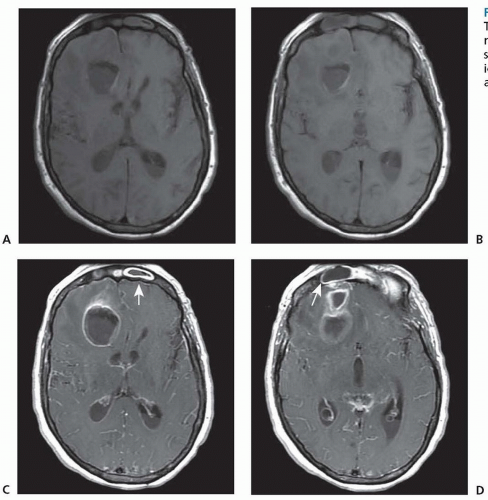
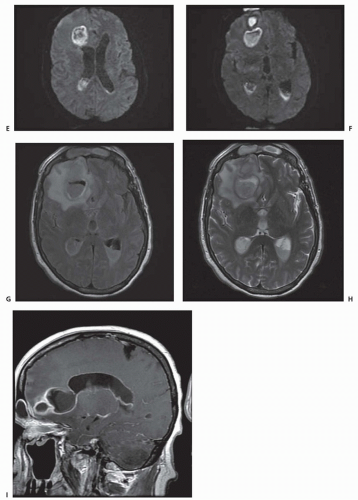
Fig. 62.2). With a mature encapsulated abscess, both contrast CT and MRI with gadolinium reveal the ring-enhancing mass with surrounding vasogenic edema. Additional MRI sequences with fluid-attenuated inversion recovery (FLAIR), T2- and diffusion-weighted images show characteristic changes (
Fig. 62.3).
The differential diagnosis, based on the appearance of lesions seen on CT or MRI, includes glioblastoma, metastatic tumor, infarct, arteriovenous malformation, resolving hematoma, and granuloma. Features supporting the diagnosis of brain abscess include gas within the center of a ring-enhancing lesion, a thinner enhancing rim (<5 mm) than with brain tumors, more regular borders with ringlike enhancement, and ependymal enhancement associated with ventriculitis or ventricular rupture (see
Fig. 62.3). MRI with diffusion-weighted image (DWI) and calculation of the apparent diffusion coefficient (ADC) may aid in differentiating tumor from abscess. With brain abscess, MRI DWI signal is hyperintense with associated low ADC values, compared to brain tumor with similar DWI appearance but high ADC. Thallium-201 brain single-photon emission computerized tomography (SPECT) may differentiate intracerebral lymphoma from
Toxoplasma encephalitis in patients with AIDS. Unlike pyogenic brain abscesses, the signal of
Toxoplasma abscesses may not be hyperintense on DWI MRI. Often, empiric treatment for
Toxoplasma can be initiated with evaluation for responsiveness to treatment using follow-up MRI imaging several weeks later.
Either CT or MRI may distinguish abscess from mycotic aneurysms or herpes encephalitis, each of which may cause similar symptoms and signs. Mycotic aneurysm, usually in the distribution of the middle cerebral arteries, may be accompanied by meningitis
in bacterial endocarditis. CT or MRI may exclude abscess, but CT angiography is often necessary to identify the aneurysm before rupture. Herpes simplex encephalitis presents with headache, fever, and an acute temporal lobe or frontal lobe syndrome. On CT or MRI, the temporal lobe is swollen, with irregular lucency and patchy contrast enhancement.
Lumbar puncture is contraindicated in patients suspected of having a brain abscess because of the clear risk of transtentorial herniation.
Both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) may be elevated in patients with brain abscess. Blood cultures are positive in patients with hematogenous dissemination as the source of infection.
Extension of the abscess to the meninges or ventricles is accompanied by findings associated with acute meningitis or ventriculitis. Rupture of an abscess into the ventricles is signaled by a sudden rise in ICP and the presence of free pus, with marked increases in CSF cell count to levels of 20,000/mm3 to 50,000/mm3. A decrease in glucose content below 40 mg/dL indicates that the meninges have been invaded by bacteria. Only rarely are CSF cultures positive.