Chapter 52D Cancer and the Nervous System
Management of Primary Nervous System Tumors in Adults
Established Treatment Strategies
Radiation Therapy
Chemotherapy
Standard Cytotoxic Chemotherapy
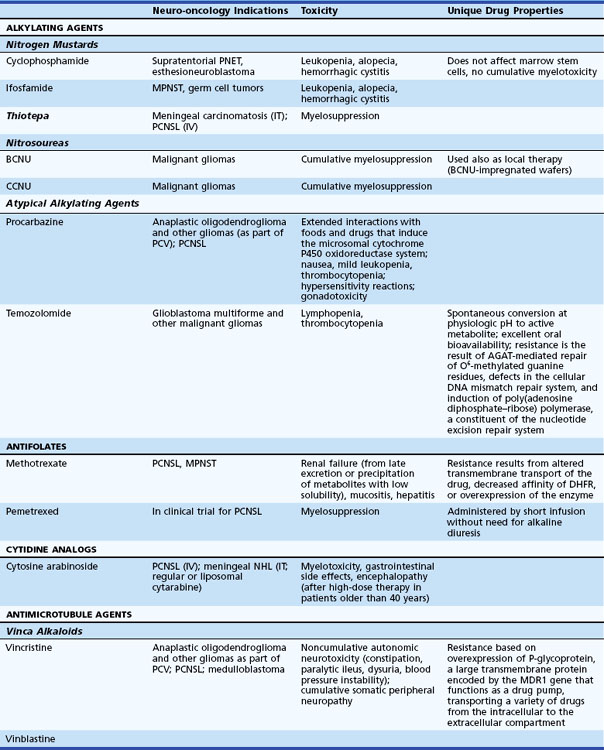
Chemotherapy is provided to most patients with malignant brain tumors. Less commonly treated are nonresected low-grade but symptomatic tumors prior to or following radiation therapy. Chemotherapy is becoming increasingly important for patients with brain lymphoma or anaplastic oligodendroglial tumors (Table 52D.1).
Table 52D.1 Cytotoxic Chemotherapeutic Agents, Applications in Neuro-oncology, Associated Toxicities, and Unique Properties
Myeloablative doses of chemotherapy followed by autologous peripheral-blood stem cell transplantation have failed to produce higher response rates in malignant gliomas when compared with conventional adjuvant chemotherapy (Finlay et al., 1996). Moreover, this approach is associated with significant treatment-related morbidity and mortality and thus has not found widespread use. Results of high-dose chemotherapy with peripheral-blood stem cell rescue in patients with chemosensitive brain tumors like anaplastic oligodendroglioma or primary central nervous system lymphoma (PCNSL) are more promising (Abrey et al., 2006).
Delivery Strategies
The BBB is the major anatomical obstacle for chemotherapy of primary brain tumors. It is composed of the endothelial cell layer of cerebral capillaries sealed by intercellular tight junctions, the vascular basal membrane, and astrocytic foot processes. Few studies have measured brain concentrations of systemically administered agents, but delivery strategies developed to circumvent the barrier include the following: (1) intrathecal administration of methotrexate, thiotepa, or cytosine-arabinoside for leptomeningeal metastases, (2) intracarotid infusion of hypertonic solutions (25% mannitol or 15% glycerol) to produce reversible opening of the BBB, and (3) biodegradable polymers impregnated with BCNU. Intracarotid infusion of hypertonic solutions, selectively used in specialized centers, produces 1 to 2 hours of barrier lysis during which hydrophilic chemotherapeutic agents such as methotrexate or cyclophosphamide are provided. The technology obligates general anesthesia and serial angiographic procedures and is associated with toxicity, including seizures and transient encephalopathy. Biodegradable polymers impregnated with BCNU increase local drug concentration without notable systemic toxicity. Dime-sized wafers of p-carboxyphenoxy (polybis) propane and sebacic acid release the chemotherapeutic agent over 7 to 10 days into tumor surrounding the resection site. The polymer-based delivery strategy is associated with median survival improvements of 2 months in patients with malignant glioma (Westphal et al., 2003). Complications include infection, wound healing impairment, brain necrosis, and cerebrospinal fluid (CSF) leak.
New Treatment Strategies
Several methods are under investigation to reduce resistance to alkylating agents. O6-benzylguanine is a potent inhibitor of AGAT that has been co-administered with alkylating agents (Quinn et al., 2005). Inhibitors of poly(adenosine diphosphate–ribose) polymerase (PARP), cell signaling enzymes implicated in cellular responses to DNA injury provoked by genotoxic stress, potentiate the effect of various chemotherapeutic agents, including alkylating compounds and inhibitors of topoisomerase 1.
Various compounds interfering with pathways regulating cell growth have been developed for numerous cancer types. Cell growth control can be attacked at different levels: growth factors, growth factor receptors, intracellular signal transducers, nuclear transcription factors, and cell-cycle control proteins. Various strategies are available to interfere with proteins or the transcription/translation of their encoding genes at each level. Modified peptides or peptidomimetics such as imatinib are molecules designed to bind to the active sites of proteins, such as the tyrosine kinase domain of growth factor receptors. Imatinib, a synthetic inhibitor of the tyrosine kinase receptors, abl and c-kit, has been of value in the therapy of chronic myelogenous leukemia and gastrointestinal stromal tumors. This has inspired the use of similar agents to target analogous brain tumor pathways. Antisense oligonucleotides injected into tumors hybridize with transcripts of growth control genes and inhibit their translation. Ribozymes degrade transcripts with high specificity. Monoclonal antibodies directly target growth-control proteins. Gene therapy may restore the function of mutated cell-cycle control proteins. A summary of new treatment strategies can be found in Box 52D.1.
Box 52D.1 Targeted Therapies for Intracranial Neoplasm*
Small-Molecule Inhibitors of Intracellular Signal Transduction Pathways
Epidermal growth factor receptor (EGFR) is an attractive target because it is commonly overexpressed or mutated. However, monotherapy with agents targeting this receptor (gefitinib, erlotinib, tyrphostin) has been disappointing (Rich et al., 2004). Molecular predictors of the rare responses have been identified (Mellinghoff et al., 2005). Inhibition of platelet-derived growth factor receptor (PDGFR) with imatinib has also been unsuccessful. This has led to the development of small-molecule inhibitors with a broader spectrum or “dual” inhibitors. AEE788 and vatalanib interfere with both EGFR and vascular endothelial growth factor receptor (VEGFR) signal transduction. Lapatinib inhibits EGFR and ErbB2, two members of the ErbB family of transmembrane tyrosine kinase receptors. These compounds are currently undergoing clinical evaluation, as are combination regimens using GFR and downstream signal transduction inhibitors (e.g., erlotinib, sirolimus).
Histone deacetylase (HDAC) inhibitors interfere with transcription. HDAC induces hyperacetylation of histones, resulting in chromatin relaxation and transcriptional activation. The anticancer properties of various HDACs have been recognized and are likely the complex result of activation of differentiation programs, cell-cycle inhibition, and induction of apoptosis in cancer cells (Johnstone, 2002). Compounds such as phenylacetate, phenylbutyrate, or valproic acid display HDAC-inhibiting properties but are unlikely to play a role as brain cancer therapeutics. Suberoylanilide hydroxamic acid (SAHA) and the fungal tetrapeptide, depsipeptide, are currently undergoing clinical evaluation in malignant gliomas.
Inhibition of angiogenesis or cell invasion represents another promising approach to brain tumor therapy. Gliomas larger than a few millimeters stimulate new blood vessel formation. This induction is affected by promoters including VEGF (hypoxia-inducible endothelial cell mitogen, vascular permeability factor), basic fibroblast growth factor (bFGF), platelet-derived growth factor, EGF, transforming growth factor (TGF), and tenascin. Endogenous inhibitors of angiogenesis include angiostatin, endostatin, thrombospondin, and heparin. Kinase insert domain receptor (KDR) and FMS-related tyrosine kinase 1 are receptors for VEGF. Bevacizumab, a humanized monoclonal antibody with murine complementarity-determining regions binding VEGF, is now approved for use in patients with relapsed glioblastoma. Several small-molecule inhibitors of VEGFR are at various stages of development. Sunitinib is already in clinical use for advanced renal cell cancer. Early experience with cediranib in glioblastoma has been promising, and a phase III study in patients with newly diagnosed disease was completed recently (Batchelor et al., 2007). Other potential therapies targeted to endothelial cells include thalidomide, interleukin (IL)-12, cyclooxygenase II inhibitors, and cilengitide, a cyclic pentapeptide inducing apoptosis of growing endothelial cells through inhibition of their αVβ3 integrin interaction with the matrix proteins, vitronectin and tenascin.
Gene therapy of brain tumors encompasses a wide spectrum of various strategies. A comprehensive review of these approaches goes beyond the scope of this chapter, and the interested reader is referred to excellent review articles (Lam and Breakefield, 2001). Viral vectors create localized inflammation while expressing transgenes that activate cytokines and chemotherapies. Transfection efficiency depends on the agent and the mode of introduction. Cells are killed not only by transfection but by the cellular reaction that damages adjacent tumor cells: the “bystander effect.” The delivery of a therapeutic gene can be enhanced by improving the vector or delivering it through infusional clysis. Ligands or antibodies targeted at receptors expressed on tumor cells (EGFR, transferrin receptor, integrin receptor) can be incorporated into the capsid of adenoviral vectors. Tumor-specific expression systems make use of the human telomerase reverse transcriptase (hTERT) promoter; hTERT is the catalytic subunit of the telomerase ribonucleoprotein and is expressed in glioma cells but not in normal glia cells. Tumor selectivity can also be accomplished by using replication-conditional viral vectors, retroviruses, or placement of genes essential to virus replication under the control of promoters that are selectively active in gliomas (e.g., nestin promoter). Currently used vector systems are either replication-defective or replication-conditional and include recombinant HSV, Ad, retrovirus, and hybrid vectors. Gene therapy delivery involves stereotactic injection into the tumor or intraoperative insertion into the wall of the resection cavity, convection-enhanced delivery, or intraarterial or intraventricular application. Nonviral strategies have made use of naked DNA, polycationic polymers, and liposomes.
Therapies based on immune-mediated strategies aim to increase immune responses to the tumor. Whether primary brain tumors suppress immune reaction, are poorly recognized by the immune system, or are protected by the immunosuppressive effects of concurrent glucocorticoid administration is uncertain. Tumor vaccination makes use of immunogenic peptides, attenuated autologous tumor cells, or dendritic cells loaded with tumor antigens. These tumor antigens create an immune reaction enhanced by irradiation, transfection with cytokine genes, or transfection with major histocompatibility complex (MHC) class II genes. The antigen can be presented in subcutaneous tissues, after which cytotoxic T cells infiltrate the site of injection as well as the brain. A “one-fits-all” immunization strategy targeting a somatic mutant of EGFR (EGFRvIII) has been tested in small phase II studies, with promising results (Sampson et al., 2009).
There are various mechanisms by which gliomas evade recognition by the immune system. Potential mediators include inhibitory cytokines (TGF-β, prostaglandin E, IL-10) and defective cytokine receptors on tumor-infiltrating T lymphocytes. Strategies rendering glioblastoma cells immunogenic have included transfection with antisense TGF-β or decorin, a TGF-β-binding and TGF-β-inhibiting proteoglycan. Cytokines can be linked to bacterial toxins as genetically engineered fusion proteins that enter the tumor cell via binding to selectively expressed receptors. A phase III clinical trial of cintredekin besudotox (IL-13 linked to Pseudomonas exotoxin) administered intracerebrally through convection-enhanced delivery failed to demonstrate a survival benefit (Debinski et al., 1998).
Oncolytic viruses are modified viruses that preferentially replicate in and destroy cancer cells. For example, ONYX-015 is a replication-competent E1B-attenuated adenovirus. E1B is a viral protein that binds and inactivates p53, a prerequisite for the virus’s ability to replicate in its host cell. Ad lacking E1B can only replicate in TP53-deficient cells. Loss of TP53 function is an early event in the pathogenesis of gliomas and thus renders them susceptible to lytic infection with this attenuated virus (Chiocca et al., 2004). Clinical trials have proven safe, but delivery systems have thus far proven insufficient.
New delivery strategies are designed to circumvent the BBB to treat malignant gliomas. Intraoperative injection of resection margins with various therapeutic agents (viral vectors, oncolytic viruses) does not depend on BBB permeability but is highly inefficient. Tissue penetration can be improved using convection-enhanced delivery. This technique requires intraoperative or stereotactic placement of infusion catheters in the wall of the resection cavity. Using microinfusion pumps, therapeutic agents are provided postoperatively over up to 96 hours. Exposure may be even further enhanced by packaging the therapeutic compound (cytotoxic chemotherapy agent, vector, antibody, etc.) into microspheres from which it is released over a modifiable period of time (Saltzman and Olbricht, 2002). Neuroprogenitor cells may deliver vectors or therapeutic genes to tumors. Animal experiments have shown that systemically administered neural stem cells home to brain tumors. Progenitor cells have been found within experimental brain tumors following injection into the contralateral cerebral hemisphere—an observation that may indicate the stem cells’ ability to track down migratory brain tumor cells (Aboody et al., 2000).
Management of Specific Brain Tumors
Neuroepithelial Tumors
Astrocytic Tumors
Noninfiltrative Tumors
Pilocytic Astrocytoma
Comprising 85% of infratentorial astrocytomas, most pilocytic astrocytomas are benign tumors located in the cerebellum and occur in the first and second decade (Burkhard et al., 2003). The remainder grow in the hypothalamus, the walls of the third ventricle, the optic pathway, and the brainstem. The tumor likely emerges from true astrocytes or subependymal precursors. Pilocytic astrocytoma, usually of the optic nerve, is the most common central nervous system (CNS) tumor associated with neurofibromatosis type 1. The tumor diagnosis is heralded by symptomatic obstructive hydrocephalus, headache, or hypothalamic-pituitary dysfunction. Posterior fossa signs include neck stiffness, head tilt, and incoordination. The masses enhance with gadolinium and appear in proximity to the ventricle or subarachnoid space. Cysts, focal hemorrhage, and calcification are described. Much of the tumor contains benign features: bipolar (piloid) cells with Rosenthal fibers in addition to microcysts surrounded by protoplasmic astrocytes and eosinophilic granular bodies. Three-quarters of patients receive surgical resection. For the unresectable case exhibiting progressive growth or symptoms refractory to treatment, involved field radiation therapy is given with a margin of 0.5 cm. Chemotherapy can be used prior to irradiation or for tumor progression. The 25-year survival rate is between 50% and 94% following surgical resection. Malignant transformation of pilocytic astrocytoma is highly unusual.
Pleomorphic Xanthoastrocytoma
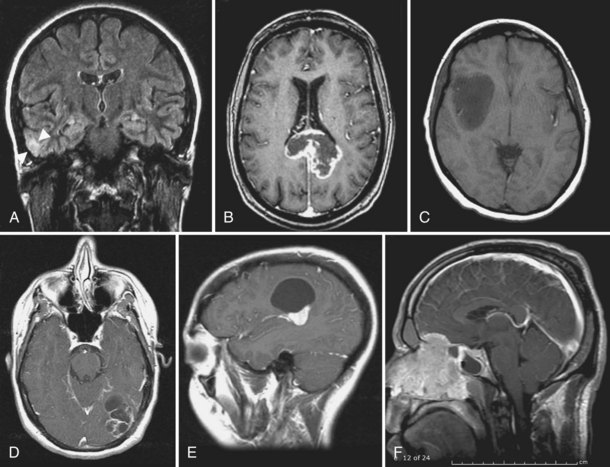
Pleomorphic xanthoastrocytoma (PXA) is a rare superficial cortical glioma (Fig. 52D.1, A). Two-thirds of cases are diagnosed before age 25. Seizures anticipate diagnosis by 3 years. The masses are located in the temporal lobes and extend into the leptomeninges and Virchow-Robin spaces. PXAs are well demarcated from surrounding tissue and may contain cysts. The solid portion of the tumor usually enhances markedly on MRI with gadolinium. On unenhanced T1-weighted images, it is hypo- to isointense. The pleomorphic cells vary in size and shape and include single and multinucleated giant cells. The large cells accumulate lipids, hence the term xanthoastrocytoma. There is some uncertainty as to the true aggressive potential of the masses; mitoses and necrosis are seen, and malignant forms have been identified. Cells of origin are probably subpial astrocytes. Complete resection is feasible in most patients, and radiation or chemotherapy is provided for aggressive or recurrent tumors. Survival is better than 80% after 5 years and 70% after 10 years, with well-resected lesions faring best (Giannini et al., 1999). Anaplastic transformation occurs in 15% to 20% of patients, for whom the natural history may be indistinguishable from glioblastoma.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree