Color-coded duplex sonography of three different segments of the internal jugular vein (IJV) using cross-sectional insonation planes: the smaller cranial segment lateral of the internal carotid artery (ICA) (top), the inflow of the thyrolinguofacial trunk (arrow) at the level of the carotid bifurcation (middle), the larger caudal segment ventral of the common carotid artery (CCA) (bottom).
Intra- and interindividual Doppler flow profiles vary widely. The venous flow is influenced by the variable vessel lumen, the contact with the nearby pulsatile carotid artery and BFV increases during inspiration due to the negative intrathoracic pressure and decreased during expiration. Usually mono- or biphasic flow profiles can be observed but an absent flow may also be seen in healthy subjects [10,13] (Figure 23.2).
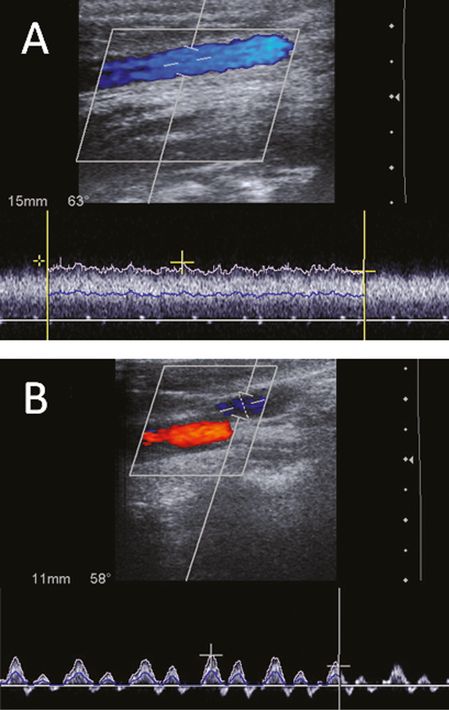
Color-coded duplex sonography in the longitudinal plane revealing different physiological flow patterns of the internal jugular vein (IJV) during normal breathing. (A) Monophasic pattern. (B) Biphasic pattern.
VVs: the VV can be best insonated in a longitudinal plane either between intervertebral segments C4/C5 or C5/C6 [11]. The vein runs parallel to the VA with an opposite flow direction. In most individuals the VV lies above the VA, in some cases a doubled VV on both sides of the artery can be observed (Figure 23.3). Caudal to the C6 segment the VV leaves the way through the transverse foramina and runs toward the brachiocephalic vein. The blood flow velocity (BFV) in this region can be higher compared to the more apical segment due to the inflow of intersegmental radicular veins of the internal VVS (Figure 23.3). The VV valve can be visualized by brightness mode (B-mode) imaging in a sagittal plane directly above the opening [8]. Further structures of the VVS are not systematically described by ultrasound, although intersegmental radicular veins are often visible (Figure 23.4). Especially in subjects with the variant of a prominent drainage via the VVS, these radicular veins, connecting the intraspinal venous system with the VVs can easily be detected by duplex ultrasound [14].
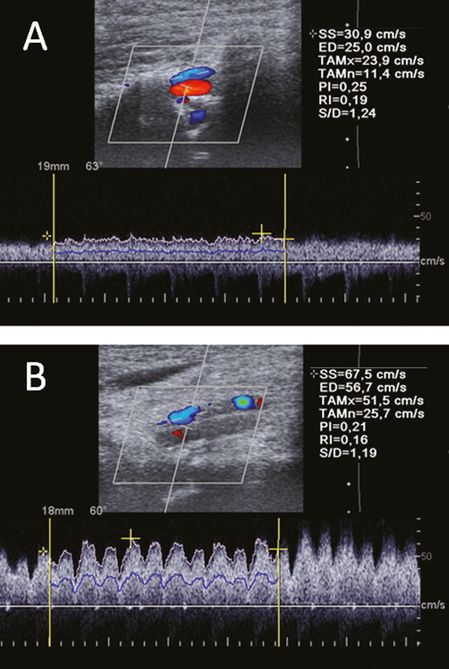
Color-coded duplex sonography of the vertebral vein in the intervertebral segment V2 between C4/C5 (A) and caudal to the C6 in the V1 segment (B) in the longitudinal plane. Note the remarkable higher blood flow velocity in the caudal segment. Time-averaged maximal velocity (TAMx) 51.5 cm/s vs. 23.9 cm/s.
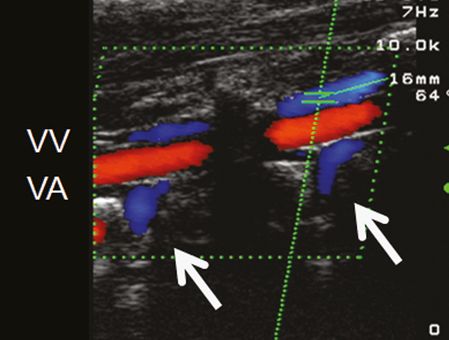
Color-coded duplex sonography of the vertebral vein (VV) in two intervertebral segments using the longitudinal plane. The VV lies above and runs parallel to the vertebral artery (VA) with an opposite flow direction. Note the intersegmental radicular veins (arrows) draining blood from the intraspinal compartment of the vertebral venous system toward the VVs.
Assessment of blood volume flow in the internal jugular veins and vertebral veins
Considering cross-sectional areas (CSAs) and time-averaged flow velocity, blood volume flow (BVF) of a vessel can be calculated. BVF in the IJVs should be assessed in the upper neck region close to the mandibular angle to measure almost exclusively intracranial BVF. First, the CSA will be measured (manually encircled) in the horizontal plane using B-mode imaging, avoiding any compression of the vessel by the probe. In a second step, the time-averaged and angle-corrected BFV over at least three heartbeats will be assessed in a longitudinal plane. For correct measurements the sample volume should enclose the whole vessel diameter (Figure 23.5, top). In cases of marked respiratory variations of CSA or BFV, the measurements can be performed during short apnea after normal exhalation. For BVF calculation, the measured CSA and BFV will be automatically multiplied by the duplex machine.
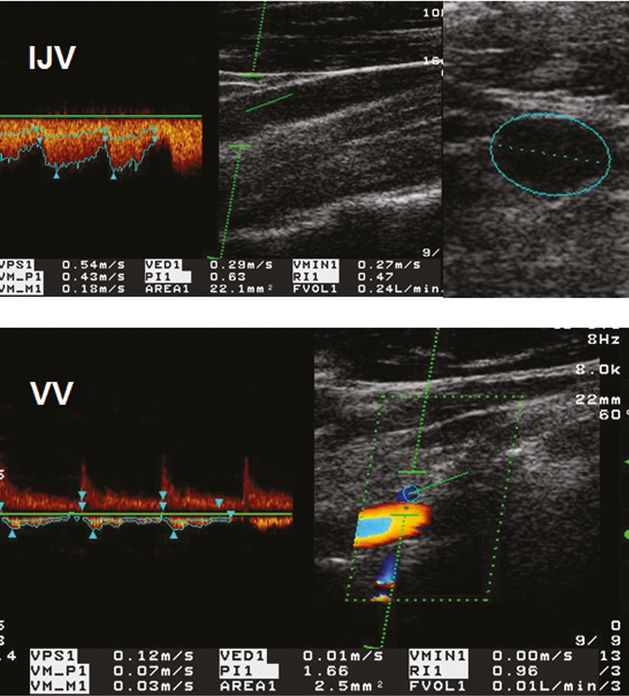
Measurement of blood volume flow (BVF) in the internal jugular vein (IJV) and vertebral vein (VV). Top: Doppler spectrum of the IJV with the time-averaged and angle-corrected blood flow velocity (VM_M1: 18 cm/s) (left), transversal B-mode scan of the IJV with a cross-sectional area (CSA) of 22.1 mm2 (right). Calculated BVF: FVOL1 is 240 ml/min. Bottom: Corresponding measurement of the BVF (10 ml/min) in the VV. The CSA (2.5 mm2) was obtained in a longitudinal plane assuming a circular vessel shape.
BVF in the VVs can be assessed either between intervertebral segments C4/C5 or C5/C6 [13]. The CSA can only be obtained in a longitudinal plane assuming a circular vessel shape (Figure 23.5, bottom). For this, the diameter of the VV will be measured and the CSA automatically calculated by the ultrasound machine. In a second step the BFV and the BVF will be analyzed in the same manner as described above for the IJV.
Internal jugular vein compression tests
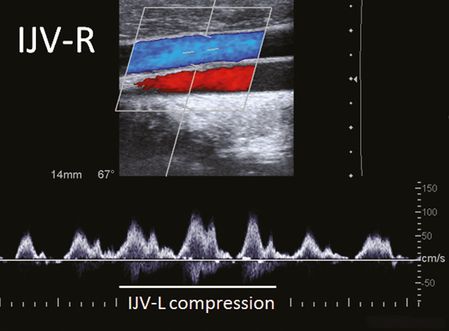
Measurement of BFV in the IJV at rest and during manual compression of the opposite IJV allows indirect assessment of the drainage anatomy in the confluens sinuum. A confluens between the SSS and StS with further drainage toward both TS exist in about 50% of a normal population, leading to a remarkable increase of blood flow in the contralateral IJV, especially during compression of the dominant IJV (Figure 23.6). An imperfect (40%) or perfect (10%) unilateral drainage of the SSS and StS into the right and left TS, however, will cause minor or no blood flow alterations during this procedure. In healthy subjects, a contralateral blood flow increase was observed in 63% and 48% during compression of the right and left IJV, respectively [6]. Bilateral IJV compression causes a significant blood flow increase in the VVs. The additional compression of the deep neck veins leads to a further rise in VV volume flow which underpins its importance as collateral pathway. However, the increase of VV BVF does not totally compensate the loss of blood flow in the compressed veins, indicating the huge capacity of the intraspinal compartment of the VVS [15].
Assessment of global cerebral circulation time
The simultaneous analysis of venous and arterial BF in the IJV and ICA allows the assessment of global cerebral circulation time (gCCT) with the aid of an echo-contrast agent. The gCCT presents a further hemodynamic parameter which is well known in conventional angiography since its introduction. In the last years it has been also used in magnetic resonance imaging as time-resolved imaging of contrast kinetic (TRICKS) [16]. The assessment of gCCT with ultrasound is based on the bolus kinetic of an echo-contrast agent injected into a cubital vein. The time difference of contrast bolus arrival in the ICA and IJV (illustrated by arterial and venous time-signal intensity curves) defines the gCCT. It was first established using a dual-channel Doppler system with a bilateral approach of two pulsed 2-MHz probes fixed near the mandibular angle using adhesive pads [17]. Later a more feasible approach was published using power mode duplex sonography with a hand-held 7.5-MHz linear array probe. Here the extracranial ICA and IJV are simultaneously insonated preferably on the side of dominant IJV in a longitudinal or axial plane. The signal gain of the power mode will be lowered until the flow is hardly visible before the contrast bolus will be injected. The time interval between the turning points of the fitted time arterial and venous intensity curves produces the most reliable CCT [18].
Physiological characteristics of venous hemodynamics
Normal values of blood flow velocity and blood volume flow
BFV and BVF in the IJVs and VVs vary widely between different subjects. Definition of normal and pathological values of BFV and BVF is less distinct compared to the arteries. Pucheu et al. described a huge range of systolic BFVs between 0 and 67 cm/s in the left and 5 and 77 cm/s in the right IJV. Furthermore, a biphasic flow pattern is frequently observed in the IJVs (57%) [10]. Hoffmann et al. first described normal BFVs in the VVs. The systolic BFV in 138 healthy subjects was 24 ± 12 cm/s with a large range between 5 and 81 cm/s and a negative correlation with age. The detection rate of the VVs in this study was in 62% bilateral and in 17% unilateral, whereas in 21% no VV could be detected [11]. The high variances illustrate the absence of cut-off values for pathological BFVs in cervical veins.
Considering BVF, an averaged BVF in both IJVs of 740 ± 209 ml/min was found in a study of 100 normal subjects ranging from 21 to 70 years without age dependency [9]. Comparable values of BVF in the IJVs were described in further studies: 839 ± 226 ml/min [19], 700 ± 270 ml/min [20] and 634 ± 246 ml/min [13]. A BVF of 40 ± 20 ml/min in both VVs was reported in a group of 23 young healthy adults [20], similar to 36 ± 34 ml/min in a larger cohort of 50 subjects [13].
Factors influencing venous hemodynamics
1. Individual different drainage types (“jugular” versus “non-jugular drainer”): in the supine position, the IJV is the main outflow pathway in 72% when considering a BVF in the IJVs of more than two-thirds of the total arterial cerebral blood flow (CBF) [13]. In 22% and 6% of the subjects the jugular BVF was less than two-thirds (453 ± 111 ml/min) or one-third (101 ± 104 ml/min) of the CBF, respectively. Differences of BVF in the VVs between the different drainage types were less significant (30 ± 25 ml/min, 52 ± 54 ml/min and 42 ± 25 ml/min, respectively). These findings illustrate that even in the supine position the VVS serves as an important drainage pathway at least in 30% of a normal population.
2. Postural venous drainage dependency: in the upright body position the IJVs collapse in varying degrees because of both increased external pressure and decreased IJV venous pressure. A postural change by 15° toward the upright body position may lead to an cessation of BFV in the IJV associated with a redistribution of venous drainage toward the VVS [20] (Figure 23.7). This phenomenon was confirmed in several studies [21,22].
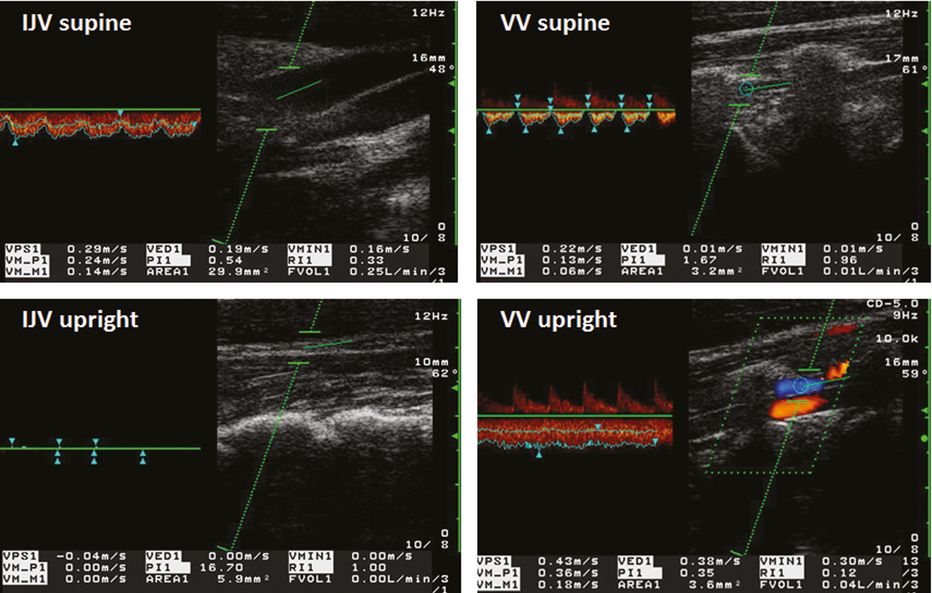
Postural dependency of venous blood flow: blood flow velocity (BFV) and blood volume flow (BVF) in the internal jugular vein (IJV) and vertebral vein (VV) in the supine (top) and upright body position (bottom). Note the decrease of the cross-sectional area (CSA; from 29.9 mm2 to 5.9 mm2), BFV (from 24 cm/s to 0 cm/s), and BVF (from 250 ml/min to 0 ml/min) in the IJV, and the simultaneous increase of CSA (from 3.2 mm2 to 3.6 mm2), BFV (from 13 cm/s to 36 cm/s) and BVF (from 10 ml/min to 40 ml/min) in the VV. Area 1 = CSA, VM_P1 = BFV (Vmean), FVOL1 = BVF.
3. Anatomical side-to-side differences of vessel diameter and drainage capacity: the CSA of the IJVs is asymmetric with a right-sided dominance in up to 80% of normal subjects [23], reflected in a significant higher BVF on the right side (438 ± 226 ml/min vs. 302 ± 194 ml/min) [9]. CSA and BFV of the VVs are similar on both sides [11].
4. Respiratory activity and intrathoracic pressure: the CSA of the IJV dilates during exhalation and narrows during inhalation due to a decrease of intrathoracic pressure. This results in a rise of BFV during inhalation and decline during exhalation [24].
5. IJV valve function: an increase of intrathoracic pressure (e.g., coughing or other during Valsalva-like maneuver) leads immediately to a closure of the valves to prevent a retrograde venous blood flow. In this situation a cessation of blood flow can be observed until the intraluminal pressure exceeds the intrathoracic pressure (Figure 23.8).
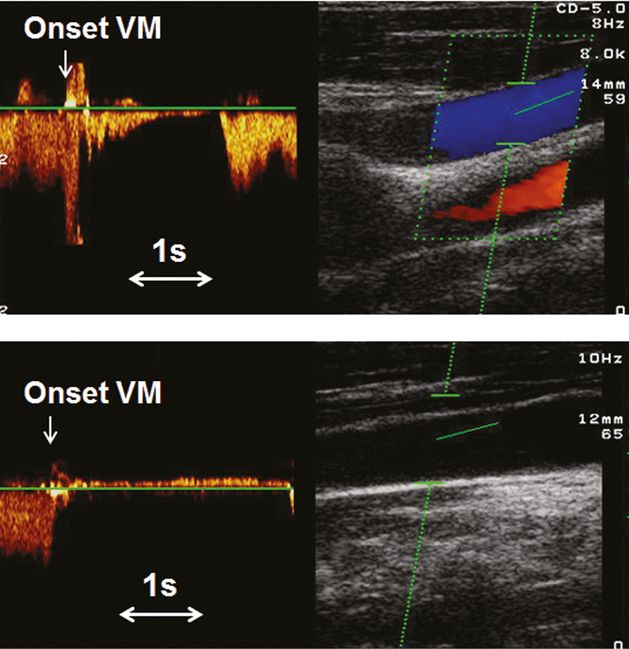
Color-coded duplex sonography and assessment of internal jugular vein valve competence. The suspension of blood flow during Valsalva maneuvers (VMs) indicates a competent valve (top), whereas the retrograde blood flow during VMs (>0.88 s) confirms valve insufficiency (bottom).
Practical implications of cervical venous ultrasound
Central venous cannulation
Duplex ultrasound is a reliable technique to facilitate catheter placement in the IJV. A systematic review of 18 randomized trials showed that the use of two-dimensional ultrasonography reduce the risk for failed catheter placement and for complications during IJV cannulation [25]. First of all, duplex ultrasound is helpful to exclude IJV thrombosis before cannulation, and to detect catheter-related thrombosis and infection [26]. Furthermore, duplex ultrasound is also helpful to detect the more suitable side (asymmetry of IJV) [27] and the optimal degree of head rotation [28] for venous puncture. We also know from ultrasound that the IJV reaches the maximal dilation in head-tilt within a few seconds and the head-down position can be done shortly before the puncture [29].
Internal jugular vein valve insufficiency
Opening and closure of the IJV valves is influenced by the intrathoracic and intraluminal hydrostatic pressure on both sides of the valve and can be directly analyzed by B-mode imaging. The valve closes with the heart cycle during diastole [30]. Competent valves hinder reflux toward the brain during increased hydrostatic pressure below the valve as it occurs during a Valsalva maneuver (VM). A venous reflux in the IJV due to valve incompetence or missing valves cannot be analyzed by B-mode imaging alone. Some authors proposed injection of agitated air and saline into the cubital vein to define internal jugular vein valve insufficiency (IJVVI) if air bubbles are detectable at rest or during VM proximal of the valve [31]. Noninvasive techniques should be preferred: a cessation or decrease of orthograde flow during repetitive VM indicates competent jugular valves, whereas a transient retrograde flow of at least 0.88 seconds discriminates with high sensitivity and specificity between a physiological transient reflux caused by valve closure from real IJVVI [32] (Figure 23.8). Almost all studies detected IJVVI in approximately 30% of healthy subjects. The high prevalence of IJVVI in patients with primary pulmonary hypertension (100%) and chronic pulmonary disease (60%) [33] suggest that chronic central/intrathoracic venous hypertension evoke IJVVI. In recent years, a high IJVVI prevalence of approximately 70–85% was described in several neurological disorders as transient global amnesia [34,35], primary exertional headache [36] and idiopathic intracranial hypertension [37]. It has been hypothesized that intracranial venous congestion facilitated by IJVVI-caused venous reflux might play a role in the pathophysiology of these diseases.
An outflow obstruction in the left brachiocephalic vein might lead to postural compression of the brachiocephalic vein causing a continuous retrograde flow only in a supine position [38].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree