Chapter 32C Clinical Neurophysiology
Transcranial Magnetic Stimulation
At the beginning of the 1980s, Merton and Morton developed the first method of noninvasive brain stimulation, transcranial electrical stimulation (TES), and this had obvious clinical application. They used a single, brief, high-voltage electric shock and produced a relatively synchronous muscle response, the motor evoked potential (MEP). The latency of MEPs was compatible with activation of the fast-propagating corticospinal tract. It was immediately clear that this method would be useful for many purposes, but a problem with TES is that it is painful owing to simultaneous stimulation of pain fibers in the scalp. Five years later, Barker and colleagues demonstrated that it was possible to stimulate the brain (and nerves as well) using external magnetic stimulation (transcranial magnetic stimulation; TMS), with little or no pain. TMS is now commonly used in clinical neurology to study central motor conduction time. Depending on the stimulation techniques and parameters, TMS can excite or inhibit the brain activity, allowing functional mapping of cortical regions and creation of transient functional lesions. It is now widely used as a research tool to study aspects of human brain physiology including motor function and the pathophysiology of various brain disorders. Because TMS can influence the brain, there have been attempts to use it as therapy, particularly when used repetitively (repetitive transcranial magnetic stimulation, rTMS). Therapeutic investigations have included stroke rehabilitation, amelioration of movement disorders, and alleviation of pain and tinnitus, but effects demonstrated so far have generally been mild. There is currently only one therapeutic indication for rTMS: medically intractable depression (Padberg and George, 2009). In this situation it can be used as an alternative to electroconvulsive therapy (ECT) to induce a remission which would then be pharmacologically maintained.
Methods and Their Neurophysiological Background
In experimental animals, a single electrical stimulus applied at threshold intensity to the motor cortex produces descending volleys in the pyramidal tract with the same velocity at intervals of about 1.5 ms. The first volley is termed the D-wave (D for direct wave), which is thought to originate from the direct activation of the pyramidal tract. The subsequent volleys are termed I-waves (I for indirect wave), presumed to be elicited by transsynaptic activation of the pyramidal tract via intrinsic corticocortical circuitry. TMS also produces both D- and I-waves in descending pyramidal neurons. In contrast to electrical stimulation that preferentially evokes D-wave first, TMS at threshold intensity often produces a corticospinal volley with I-waves but no early D-wave (Ziemann and Rothwell, 2000). This finding suggests that TMS activates pyramidal neurons indirectly through synaptic inputs but does not activate them directly, presumably because of the direction of its current flow. Standards for the use of TMS and review of side effects have been published (Rossi et al., 2009).
Motor Excitability Measurements
Silent Period and Long-Interval Intracortical Inhibition
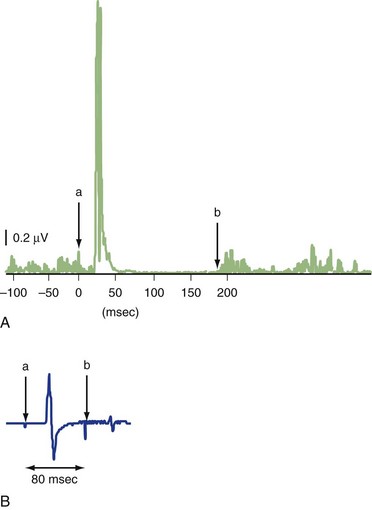
The silent period (SP) is a pause in ongoing voluntary electromyography (EMG) activity produced by TMS (Fig. 32C.1, A). The SP is usually measured with a suprathreshold stimulus in moderately active (usually 5%−10% of maximal voluntary contraction) muscle. SP duration is usually defined as the interval between the magnetic stimulus and the first recurrence of rectified voluntary EMG activity (Chen et al., 1999; Curra et al., 2002). While the first part of the SP is due in part to spinal cord refractoriness, the latter part is entirely due to cortical inhibition (cortical silent period, CSP). If a second suprathreshold test stimulation (TS) is given during the SP following suprathreshold conditioning stimulus (CS) (usually 50−200 ms after the first stimulus), its MEP is significantly suppressed (long intracortical inhibition; LICI) (see Fig. 32C.1, B) (Chen et al., 1999; Curra et al., 2002). SP and LICI appear to assess GABAB function, although other drugs affecting membrane excitability or dopaminergic transmission also influence SP. Although LICI and SP share similar mechanisms, they may not be identical, because they are affected differently in various diseases (Berardelli et al., 1996).
Short-Interval Intracortical Inhibition and Intracortical Facilitation
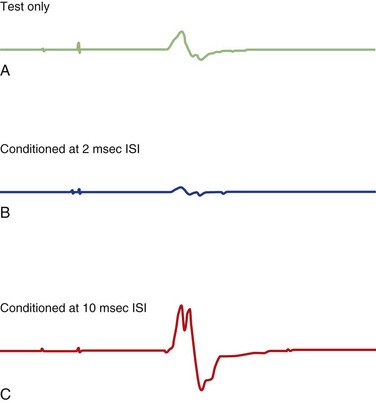
Various inhibitory and excitatory connections in the motor cortex can be evaluated by TMS using a paired-pulse technique. A subthreshold CS preferentially excites interneurons, by which MEPs from a following TS are suppressed at interstimulus intervals (ISIs) of 1 to 5 ms (short intracortical inhibition; SICI for such inhibition at short intervals) or facilitated at ISIs of 8-20 ms (intracortical facilitation; ICF) (Fig. 32C.2) (Ziemann, 1999; Ilic et al., 2002). SICI and ICF reflect interneuronal activity in the cortex. SICI is likely largely a GABAergic effect, especially related to GABAA receptors, whereas ICF is largely a glutamatergic effect. SICI can be divided into two phases, with maximum inhibition at ISI of 1 ms and 2.5 ms. SICI at 1 ms ISI is presumably caused either by neuronal refractoriness resulting in desynchronization of the corticospinal volley or by different inhibitory circuits; SICI at ISI of 2 ms or longer is most likely a synaptic inhibition.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree