6 Chiari malformations include a group of complex anomalies of the hindbrain with different etiology, pathophysiology, and clinical feature. These malformations range from the simpler to the more complex varieties of presentation, signifying their stages of appearance during embryologic differentiation and development (Table 6.1). Various management modalities have been put forth to treat this entity. Over the years there has been a significant advance in understanding the pathophysiology, diagnostic modalities, and management strategies. It was John Cleland (1835–1925), a Scottish anatomist, who first described a case of hindbrain malformations in 1883.1 In his article, “Contribution to the Study of Spina Bifida, Encephalocele, and Anencephalus,” Cleland described the pathophysiologic findings in nine infants at autopsy and two chick embryos.1 Later Hans Chiari (1851–1916), an Austrian pathologist who practiced medicine in Vienna, Prague, and Strasbourg, published his work in 1891.2,3 Chiari described the type I malformation as “peg-like elongation of tonsils and medial divisions of the inferior lobes of the cerebellum into cone-shaped projections which accompany the medulla oblongata into the spinal canal,” while sparing the medulla.2 In 1894, Julius Arnold (1835–1915), who studied under Virchow and Friedreich, described a case of an infant with spina bifida and elongation and descent of the inferior part of the cerebellum into the spinal canal.2,4 In 1907, Schwalbe and Gredig, while working in Arnold’s laboratory at Heidelberg, described four cases with meningomyelocele and alterations in the brainstem and cerebellum, and used the term “Arnold-Chiari.”1,3,4 With the advent of magnetic resonance imaging (MRI), the intracranial anomalies associated with various Chiari malformations have become much clearer, and MRI became the imaging modality of choice in the diagnosis of Chiari malformations and the associated syrinx. MRI had been used to quantify the extent of tonsils below the foramen magnum. Aboulezz et al5 quantified the extension of tonsils below the foramen magnum based on MRI findings. The extension of tonsil below the foramen magnum is considered normal if it is less than 3 mm, borderline if it is between 3 and 5 mm, and clearly pathologic when it exceeds 5 mm.5 According to ]Barkovich et al,6 an MRI demonstration of tonsillar ectopia of <2 mm is probably of no clinical significance in the absence of syringomyelia. Further studies have shown that a small posterior fossa predisposes to hindbrain overcrowding, especially in patients with Chiari type I malformations.7 Further advances in MRI techniques, such as cine-MRI, greatly helped to study the alterations in the cerebrospinal fluid (CSF) flow at the foramen magnum7 and became a useful tool to evaluate the results of posterior fossa decompression radiologically. The embryologic timing of the occurrence events responsible for the development of the Chiari malformation is not very clear. Daniel and Strich8 suggested that the abnormalities might develop early in the embryonic life. Based on their observation, they postulated that failure to form pontine flexure at the 6th week of fetal life may be one of the factors accounting for the marked elongation of the brainstem. They observed that in the initial part of fetal life (about 37 days), the length of hindbrain relative to forebrain is very great, and only after the formation of the pontine flexure does the hindbrain shorten. They also postulated the mechanism for the development of the kink. The cervical flexure is produced in the fourth week of fetal life and undone during the second month.8 If there is an elongated hindbrain during this process of straightening out the cervical flexure, it may result in a possible kink. The caudal displacement of the cerebellum and brainstem would result in an enlarged foramen magnum and a smaller posterior fossa that permits the tentorium to have a low insertion. However, this theory fails to explain the other associated spinal and cranial abnormalities. Table 6.1 Description of Chiari Malformations
Congenital Chiari Malformations
♦ Pathogenesis of Chiari Malformations
Developmental Arrest
| Chiari type I |
• Tonsillar herniation greater than 5 mm below the foremen magnum • Most commonly seen clinical entity • Also referred to as adult-type Chiari malformation • Syringomyelia common |
| Chiari type II |
• Caudal descent of the cerebellar vermis along with brainstem and fourth ventricle • Associated with myelomeningocele • Hydrocephalus is commonly seen |
| Chiari type III |
• Rarest and most severe form • Occipital or high cervical encephalocele containing herniated cerebellar or brainstem tissue |
| Chiari type IV |
• Marked hypoplasia or aplasia of the cerebellum • Associated tentorial hypoplasia |
Crowding Theory
The study by Marin-Padilla and Marin-Padilla9 showed that administration of vitamin A to pregnant hamsters induces types I and II Arnold-Chiari malformations. According to this theory, the primary defect is mesodermal, involving the cranial base. This has resulted in a small and short posterior fossa that is inadequate to contain the developing neural structures of the region.9 The primary paraxial mesodermal defect can affect the embryo at any time relative to closure of the neural folds.9 According to Sarnat,10 the crowding theory postulated by Marin-Padilla and Marin-Padilla would only impart a significant influence in late gestation. Nishikawa et al11 quantitatively measured the volume of the posterior cranial fossa and volume of the neural structures in the posterior cranial fossa. Their results strongly suggest the overcrowding hypothesis. Overcrowding of the neural structures (normal-sized hindbrain) in the underdeveloped posterior cranial fossa induces a downward herniation of the hindbrain.
Hydrodynamic Mechanisms
According to Gardner,12 a balance exists between the pulsatile flow in the lateral ventricle choroid plexus and the fourth ventricle choroid plexus. The final position of tentorium is determined by the hydrodynamic effects of the anterior and posterior choroid plexus. According to Gardner, if the caudal end of the neural tube ruptures, the competing effect of the posterior choroid plexus is largely lost. The relatively increased effectiveness of the anterior choroid plexus causes the lateral ventricle to overdistend.
Chiari malformations result from imbalances between the pulsating choroid plexus in the fourth ventricle and in the lateral ventricle. Overactive supratentorial pulsations might cause tentorial migration, resulting in the development of a Chiari malformation.12 This theory also fails to explain the occurrence of the other associated anomalies. The hydrodynamic theory does not explain the associated supratentorial anomalies.
Oligo–Cerebrospinal Fluid or the Unified Theory
This theory was proposed by McLone and Knepper.13 According to this theory, distention of the embryonic ventricular system is critical to normal development of the brain. If during the early part of the fetal development the presence of an open neural tube defect leads to escape of the CSF and fails to distend the embryonic ventricular system, the small posterior fossa is a consequence.
Neuroschisis
According to Padget,14 splitting open the neural tubes results in escape of the fluid from the neural tube and the formation of “neuroschistic blebs.” These blebs might heal totally, lead to formation of a spina bifida occulta, or might rupture with the eversion of neural cleft margins, leading to an open spinal lesion. Padget explained that a Chiari malformation is a sequel to a neural cleft, which allows fluid to escape from the neural tube. Embryonic microcephaly results, and the cerebellar primordia prematurely approximate and fuse in the posterior fossa, which is already small. Further development of the cerebellum in a small posterior fossa results in herniation out of the foramen magnum with subsequent obstruction of the fourth ventricle, producing hydrocephalus, whereas the microcephaly leads to folding and fusion at the level of the midbrain, resulting in aqueduct stenosis.
Pulsion Theory
According to this theory, fetal hydrocephalus pushes the contents of the posterior fossa downward from above.12,15–17 This theory was originally proposed by Chiari.10 This theory cannot explain the occurrence of the Chiari malformations without hydrocephalus and other associated anomalies.
Neuroectodermal-Mesodermal Spatial Dyssynchrony
Jennings et al18 hypothesized that the etiologic event responsible for the Arnold-Chiari malformation is the caudal displacement of the site of the initial fusion of the neural folds. They postulated that the normal zone of fusion at the third and fourth somites is displaced caudally below the third to fifth somite pairs, thus displacing the area of formation of the cervicomedullary junction. Their observations were based on the study of a 130-day-old human fetus with associated Arnold-Chiari malformation and thoracolumbar myeloschisis, which revealed evidence of neuroectodermal mesodermal spatial dyssynchrony.
Traction Theory
This theory, proposed by Penfield and Coburn19 in 1938, states that traction due to tethering of the spinal cord at a lower level prevents upward migration during early development, while the cerebellum and brainstem are pulled down as the vertebral column grows. Goldstein and Kepes20 tested the effect of spinal cord tethering on the development of Arnold-Chiari malformation in experimental animals and could not find any evidence of the malformation in the experimental animals that survived and reached adulthood. It is very unlikely that traction forces affect the cervical spinal cord and medulla.20 Traction theory fails to explain the lack of other observations, such as the medullary kink, and the spinal cord is not always tethered in patients with Chiari malformations.
Molecular Genetic Hypothesis
Sarnat21,22 postulated that Chiari malformation II is a disturbance of rhombomeric segmentation and ectopic expression in the embryonic hindbrain due to genetic mutation. HOX family genes in particular are implicated because they not only program hindbrain segmentation but also are important in the development of basioccipital, exoccipital, and supraoccipital bones of paraxial mesoderm origin.23 The same group studied several markers like vimentin in the ependyma of patients with Chiari II malformations.10 The results of the study showed that vimentin is focally upregulated only in areas of dysgenesis. Based on their observation, they speculated that the focal upregulation of vimentin could be a secondary event as a result of defective expression of another gene. This observation may also support a molecular genetic hypothesis.
♦ Classification of Chiari Malformations
Chiari 0
Iskandar et al24 in 1998 reported resolution of syringohydromyelia without hindbrain herniation in five patients after posterior fossa decompression. They referred to such cases of syringomyelia without hindbrain herniation as the “Chiari 0” malformation. Later the same researchers showed that the contents of the posterior fossa are compromised and distorted in these patients with syringohydromyelia without hindbrain herniation.25 They suggest that a smaller than normal posterior fossa is just enough to compromise CSF egress out of the cranium.25
Milhorat25 feels that cerebellar tonsils are paramedian structures, and on midsagittal MRI scans minimal tonsillar herniation might be missed. Milhorat et al,7 in their study of 364 patients with Chiari I malformation, showed that in these patients the CSF volume in the posterior fossa is significantly smaller, and tonsillar herniation of less than 5 mm does not exclude the diagnosis of Chiari I malformation. Milhorat25 suggests that a better description of such cases with “Chiari 0” malformation would be “borderline Chiari malformations.” Rather than classification issues, one may have to investigate these patients without significant tonsillar herniations using cine-MRI7 scans to document disturbance in the CSF circulation in the posterior fossa before subjecting them to hindbrain decompression.
Chiari I
Chiari I malformation is characterized by caudal displacement of the cerebellar tonsils by more than 5 mm into the cervical spinal canal below the plane of the foramen magnum. According to Milhorat et al,7 tonsillar herniation of less than 5 mm does not exclude the diagnosis of Chiari I malformation. This is the most commonly seen clinical entity and is described as synonymous with tonsillar herniation. It is usually referred to as adult-type Chiari malformation due to its incidence in the second or third decade of life.7 The exact incidence of Chiari I malformations is not known. Meadows et al26 reviewed 22,591 patients who underwent MRI of the head and cervical spine. Of these only 175 patients (0.8%) had Chiari I malformation.
Associated Anomalies
Craniovertebral anomalies are frequently seen in patients with Chiari malformation.27,28 In the study by Milhorat et al,7 the most common bony anomalies were reduced height of the supraocciput (84%), increased slope of the tentorium (82%), reduced length of the clivus (49%), retroflexion of the odontoid (26%), and basilar invagination (12%). Tubbs et al29 graded the posterior angulation of the odontoid process in patients with Chiari I malformation and found that higher grades of posterior angulation are more often associated with syringomyelia. Other commonly seen osseous abnormalities are platybasia, midline occipital keel, remnants of proatlas, atlantoaxial dislocations, Klippel-Feil anomaly, empty sella, and clival concavity.30,31
The incidence of syringomyelia associated with Chiari malformation varies between 50% and 75%.7,32–35 The common location of the syrinx is cervical followed by the thoracic spinal cord (Fig. 6.1),35 occasionally with holocord syrinx (Fig. 6.2). A thickened dural band posterior to the arch of C1 may be frequently encountered during surgery.35 Chiari I malformation is associated with hydrocephalus in 7 to 10% of cases.7,35 An occasional patient might show an elongated brainstem with medullary kinking (Fig. 6.3).
Chiari 1.5
This group comprises patients with tonsillar ectopia as seen in Chiari I malformation. In addition, they exhibit caudal descent of the brainstem (Fig. 6.4). This has been specifically referred to as “Chiari 1.5”36,37. No other sign or symptom was peculiar for Chiari 1.5 malformations.37
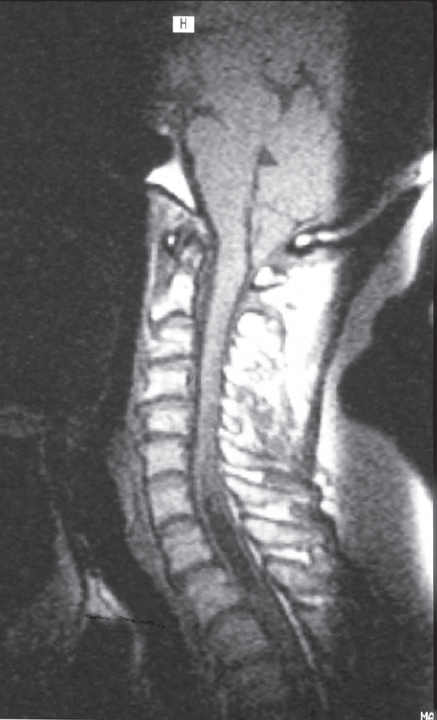
Fig. 6.1 Sagittal T1-weighted magnetic resonance imaging (MRI) of the craniocervical junction showing the brainstem kink and the herniating cerebellar tonsils across a funnel-shaped foramen magnum. There is apparently occipitalization of atlas.
Chiari II
Chiari II malformation is characterized by caudal descent of the cerebellar vermis along with the brainstem and fourth ventricle.
Associated Anomalies
Myelomeningocele (MMC) is nearly always associated with Chiari II malformation.38 Luckenschadel skull is an ossification disorder in which the fetal skull appears fenestrated. It is almost always associated with Chiari II malformation and MMC.39 Other anomalies associated with Chiari II malformation are petrous scalloping, enlargement of the foramen magnum, fenestrations of the falx, falx hypoplasia, and hypoplastic tentorium with wide incisura.40 The inion is lower, and there may be an occipital keel with less frequency of basilar invagination. The upper cervical spine shows Klippel-Feil anomaly with hypoplastic posterior arch of C1 and scalloped dens.41
Other abnormalities associated with Chiari II malformation, as detected in MRI scans, are hypothalamic adhesions (48.6%), low anterior commissure (38%), abnormalities of the corpus callosum and hippocampal commissure (57%), callosal ridge (60%), cortical posterior stenogyria (72%), gray matter heterotopias (19%), hippocampal abnormalities (85%), and atypical sulcation of the adjacent temporomesial cortex (93%).38 The midbrain is elongated with a shortened quadrigeminal plate. Peaking is seen with fusion of the colliculi (tectal beaking). Callen et al42 observed tectal beaking in 66% of patients with Chiari II malformation on prenatal sonography. The caudal displacement of the lower brainstem may create a medullary kink in the cervical canal.43
Hydrocephalus is seen in 90% of patients with Chiari II malformation.44 The other ventricular abnormalities found in patients with Chiari malformation II are “shark-tooth deformity” of the third ventricle, colpocephaly, beaking of the frontal horns, severely deformed lateral ventricles (although they may also be normal), small fourth ventricle, and small aqueduct.45 The vast majority of type II patients exhibit syringohydromyelia, sometimes with exophytic components mimicking arachnoid cyst36,46 (Fig. 6.5). In approximately 6 to 15% of cases, there is an associated spilt-cord malformation.47,48
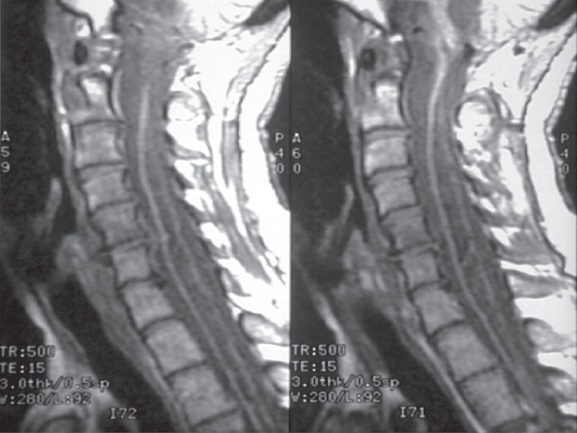
Fig. 6.2 A cervicothoracic syrinx seen in a Chiari malformation in this sagittal T1-weighted MRI. Most often this type of syrinx disappears after decompression at the foramen magnum.
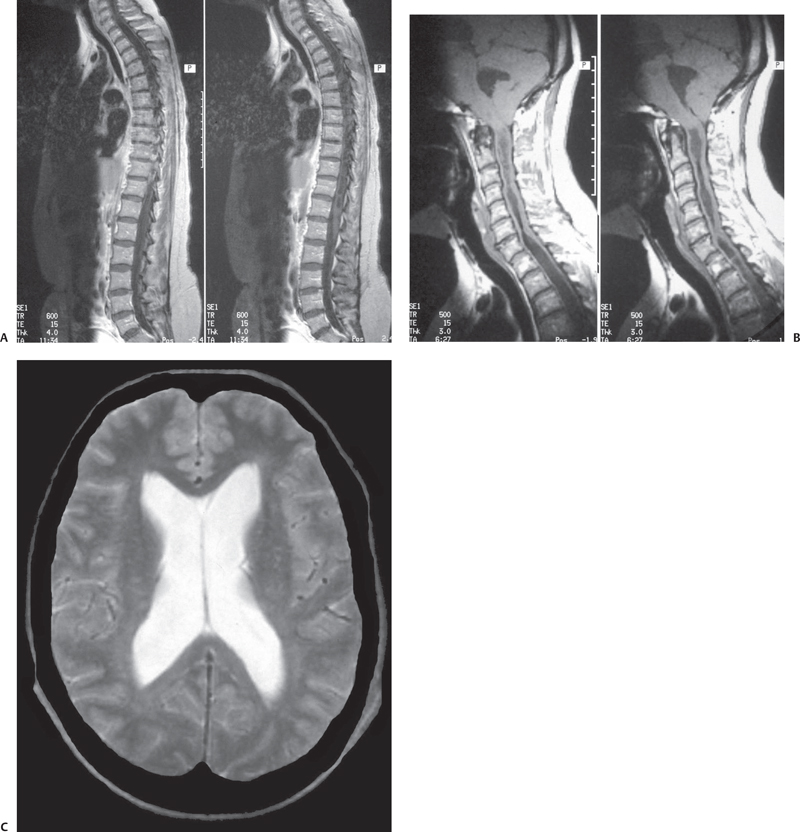
Fig. 6.3 (B) Sagittal T1-weighted MRI of the craniocervical junction shows tonsillar herniation with an elongated fourth ventricle. (A,B)There is holocord syringomyelia with irregular cavitations. T2-weighted brain sections demonstrate ventricular dilatation in the same patient. (C) T-2 weighted brain sections demonstrate ventricular dilation in the same patient.
Chiari III
Type III is the rarest and most severe form of all the Chiari malformations. It is characterized by occipital or cervical encephalocele along with the above intracranial abnormalities seen with type II malformation.49,50 The sac of encephalocele contains dysmorphic and ischemic neural elements (Fig. 6.6).
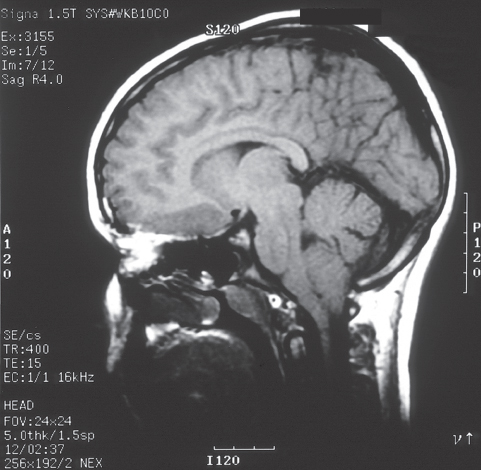
Fig. 6.4 T1-weighted MRI of the brain of a 13-year-old boy showing tonsillar ectopia. A slight peaking appearance of the brainstem is also seen.
Other cranial abnormalities, such as small posterior fossa, low tentorial attachment, scalloping of the clivus, tectal beaking, and dysgenesis of severely deformed corpus callosum, are seen.49
Chiari IV
This type is characterized by marked cerebellar hypoplasia or aplasia and tentorial hypoplasia. There is no hindbrain herniation.
♦ Clinical Presentation
Of all the Chiari malformations, type I is most commonly encountered. After the advent of MRI scans, several atypical, silent, or incidental entities have been reported. At Louisiana State University Health Sciences Center in Shreveport, Louisiana, the experience with these malformations amounts to 95 patients, of whom approximately 20% were children, although 9.5% presented after 55 years of age. There was female preponderance: 72% were females and 28% were males.
Type I Malformations
The signs and symptoms in patients with Chiari I malformation are generally related to the compression of the neural structures by the herniated tonsils51 and by the presence of an associated syrinx. Clinical manifestations can be grouped into cranial nerve dysfunction, long tract deficits, and cerebellar dysfunction.
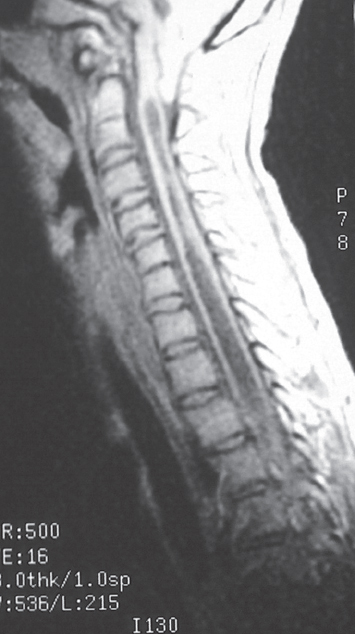
Fig. 6.5 A more severe descent of the cerebellum along with a low-lying fourth ventricle is seen in this T1-weighted MRI. Note the syringomyelia starting right at the level of spinal cord compression by the tonsils. The brainstem is showing kink with compression of medulla.
Suboccipital headache is one of the common presentations of the Chiari I malformation. These headaches are usually exaggerated by physical exertion and the Valsalva maneuver.7 Ocular disturbances such as retro-orbital pain, floaters or flashing lights, blurred vision, photophobia, and diplopia are frequently reported by patients.7 Otoneurologic disturbances such as dizziness, disequilibrium, tinnitus, decreased hearing or hyperacusis, vertigo, and oscillopsia are commonly seen.7,52 Downbeat nystagmus is characterized by slow upward drift and fast downward phases.53 One of the common causes for downbeat nystagmus is Chiari malformation.53
Lower cranial nerve, brainstem, and cerebellar disturbances are seen in up to 52% of patients.7 The most common symptoms are dysphagia, sleep apnea, dysarthria, and incordination.7 Objective signs are absence or impairment of the gag reflex, vocal cord paralysis, facial hypoesthesia, dysmetria, and truncal ataxia. Long tract signs include posterior column dysfunction and pyramidal weakness.
Patients might present with clinical features due to an associated syrinx. The signs and symptoms vary with the location and extent of the syrinx. The most common symptoms are muscular weakness, paresthesias, dysesthesia, nonradicular segmental pain, analgesia, spasticity, trophic phenomenon, and poor position sense.7 Dissociated sensory loss entails loss of pain and temperature sensation with preservation of light touch. An expanding syrinx disrupts the decussating sensory fibers passing from the dorsal horn to the opposite lateral spinothalamic tract. Disassociated sensory loss is not always a necessary finding for the diagnosis of syringomyelia.54 These patients might have features of both upper motor neuron and lower motor neuron dysfunction.
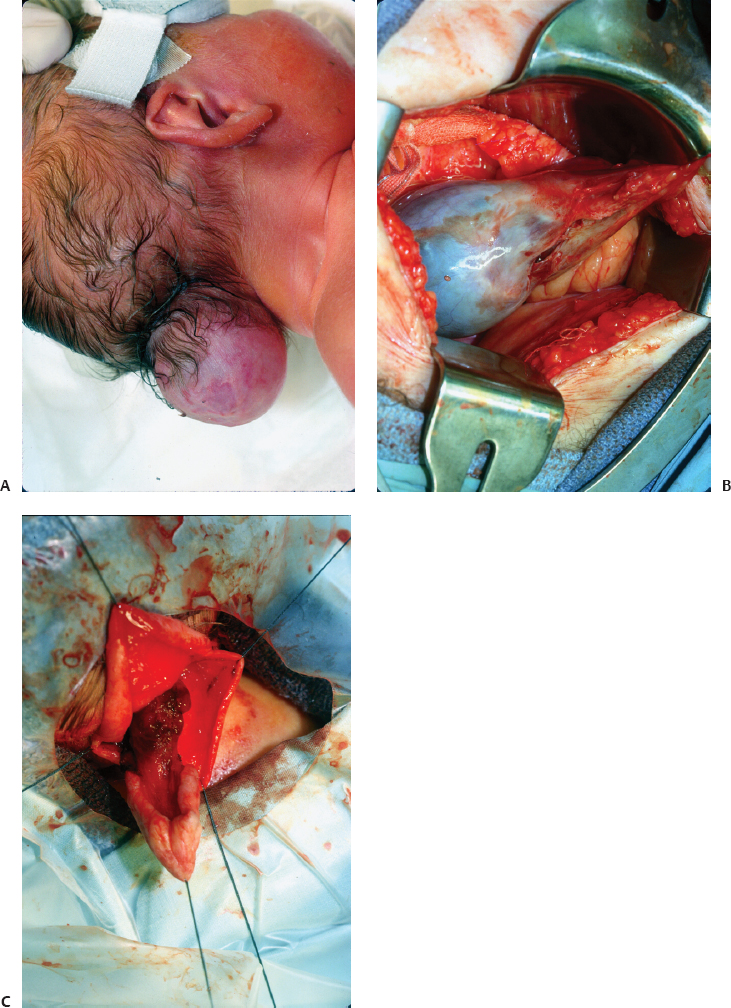
Fig. 6.6 (A–C) An occipital encephalocele in a young child. Intraoperative pictures show the atrophic neural elements inside the meningeal sac and after excision.
The presenting features in children sometimes differ from the presenting features in adulthood. Children under 3 years of age might present with oropharyngeal dysfunction, and slightly older children (3 to 5 years of age) might present with scoliosis, headache, or neck pain.55
Type II Malformations
The first sign in these patients would be an MMC, taking into account that Chiari II malformation is universally associated with MMC.45 Infants and children younger than 2 years of age present most frequently with cranial nerve and brainstem dysfunction, and the most common and potentially fatal symptom in these age groups involves respiratory difficulties.45 Other symptoms in infants include hypotonia, opisthotonus, nystagmus, weak cry, and developmental delay.45 Symptoms in older children are not life threatening and tend to be more insidious, and features of cervical myelopathy are the hallmark finding in these age groups.45 Older children can also present with ataxia, occipital headache, neck pain, and features of syringomyelia45
Type III Malformations
These children usually present with breathing difficulty, swallowing problems, seizures, developmental delay, weakness and hyporeflexia in the upper extremities, and spasticity in the lower extremities.56,57
♦ Diagnostic Imaging
Magnetic resonance imaging is the diagnostic test of choice. It provides simultaneous visualization of the cervical spine and the entire neuraxis. Syringomyelia and syringobulbia are easily demonstrated. MRI facilitates the identification of the venous anomalies of the posterior fossa. The level of the torcular and the transverse sinus are important to plan the posterior fossa decompression. In cases of Chiari III malformation, MRI enables the surgeon to identify the amount of neural tissue in the encephalocele and the position of the brainstem. The anomalies associated with various Chiari malformations were described above.
Dynamic studies are useful to demonstrate CSF flow around the foramen magnum, and can show obstruction by hindbrain structures or evidence of syringomyelia. Cine-MRI is helpful in demonstrating a CSF flow disturbance at the foramen magnum.7 McGirt et al58 demonstrated that normal preoperative hindbrain CSF flow is an independent risk factor for treatment failure after decompression for Chiari I malformation regardless of the degree of tonsillar ectopia. In another study the presence of decreased CSF flow both ventral and dorsal to the cervicomedullary region was associated with improved response to posterior fossa decompression.59 Flow studies and cine-mode MRI are useful for postoperative evaluation following decompressive surgery. Flow obstruction, decreased velocities, and a shorter period of caudal CSF flow in the foramen Magendie and foramen magnum in patients with more than 5 mm of tonsillar herniation are reported.60
♦ Brainstem Auditory Evoked Potentials
Zamel et al61 studied the role of brainstem auditory evoked potential (BAEP) monitoring during posterior fossa decompression. They showed that a predominant improvement in central conduction in most of their patients occurred during the period of bony decompression. Similarly, Anderson et al62 found that improvement in conduction velocity occurs after bone decompression and division of the dural band. The predictive value of the improvement in BAEP needs to be assessed further in larger studies to establish its role in the surgical management of Chiari malformations.
♦ Surgical Treatment and Outcome
Chiari I Malformations
With the advent of MRI an increasing number of patients are being diagnosed with asymptomatic Chiari I malformation. An important controversy in the management of asymptomatic Chiari I malformation concerns the role of prophylactic surgery in asymptomatic patients. Novegno et al51 studied the natural history of Chiari I malformation. Their findings suggest that a conservative approach is indicated in both asymptomatic and slightly symptomatic patients, with periodic follow-up. Haroun et al63 reported that in their survey of the members of the pediatric section of the American Association of Neurological Surgeons, the majority of the respondents rejected the routine use of prophylactic surgery for Chiari I malformation.
Cerebrospinal Fluid Diversion Procedures
In patients with hydrocephalus, the CSF diversion procedure is considered the best initial treatment option. Ventriculoperitoneal (VP) shunting is the most common CSF diversion procedure. Recently, endoscopic third ventriculostomy has been shown to be efficacious in patients with hydrocephalus associated with Chiari I malformation.64 Following the CSF diversion procedure, if the symptoms persist or if the syrinx does not show improvement or enlarges, then one needs to direct one’s attention to a Chiari decompression.
Posterior Fossa Decompression
♦ Surgical Technique
In brief, this operative procedure is performed with the patient in the prone position. A midline skin incision is made from 2 cm above the inion up to C2. The incision is deepened down to the bone along the midline avascular plane. Self-retaining retractors are placed, and the musculature is gently retracted. At the level of the superior nuchal line, the suboccipital muscles are divided with a thin rim attached to the bone. The muscles are separated subperiosteally from the underlying occipital squamous bone. Inferiorly the muscular attachment to the retractor arch of the atlas is detached by sharp dissection. Weitlaner is placed superiorly and inferiorly. While separating the muscle and soft tissue, one needs to be extremely careful to avoid injury to the vertebral artery. The pulsations of the vertebral artery can be seen and felt. Any brisk bleeding from the venous plexuses can be controlled with Gelfoam, and it is a warning sign that the vertebral artery is nearby. We prefer a small craniotomy (5 cm). The foramen magnum rim is removed with either a highspeed drill or a Kerrison punch. The dural arch of C1 is excised. The dura is opened in a Y-shaped manner. At this stage, the microscope is brought into the field. The arachnoid is opened separately. The tonsils are usually tongue-like and smooth, very often pulling down the posterior inferior cerebellar artery along with them into the spinal canal. Care has to be taken to avoid injury to the artery while dissecting the tonsils from the arachnoid adhesions (Fig. 6.7). We routinely shrink the tonsils with bipolar coagulation. A fascia lata graft is used for duraplasty, and the dura is closed in a watertight manner.
♦ Outcome
In the vast majority of centers, the procedure has yielded good results. Postoperative MRI established the efficacy of this method in providing patent CSF pathways around the craniocervical junction.65,66 Park et al33 found improvement in all their patients over long-term follow-up. Klekamp et al67 reported an 87% decrease in the size of the syrinx in their series of 131 patients, whereas Garcia-Uria et al68 found 50% improvement and 75% stabilization over a follow-up period of 5 to 10 years, respectively. Fischer69 reported resolution of the syrinx in 93% of cases in a literature survey. Anderson et al62 reported postoperative improvement in BAEPs following craniocervical decompression. Not only did syringomyelia and symptoms of hindbrain compression improve, but also scoliosis, which is seen with long segment syringomyelia, responded to posterior cranial fossa (PCF) decompressive surgery.70,71
♦ Controversies
There are several controversies regarding intradural procedures such as dissection of the arachnoid overlying the tonsils, shrinkage of the tonsils by bipolar coagulation, and subpial resection of the tonsils. A recent study found that tonsillar management has no significant effect on improvement of syringomyelia.72
In some studies the results are good without any Intradural manipulation.73–75 Genitori et al74 studied the role of posterior fossa bony decompression without dural opening in the management of symptomatic children affected with Chiari type I malformation. With this approach they achieved improvement in symptomatology in a high percentage of cases (97.2%). A similar strategy was adopted by James et al.76 Zamel et al61 described their experience with neurophysiologic intraoperative monitoring of BAEPs during posterior fossa decompression surgery for the management of Chiari I malformation. Their findings suggest that posterior fossa decompression with bone removal alone significantly improves conduction time in most pediatric patients with Chiari I malformation. The use of duraplasty facilitated a small improvement in conduction time in only 20% of the patients.61
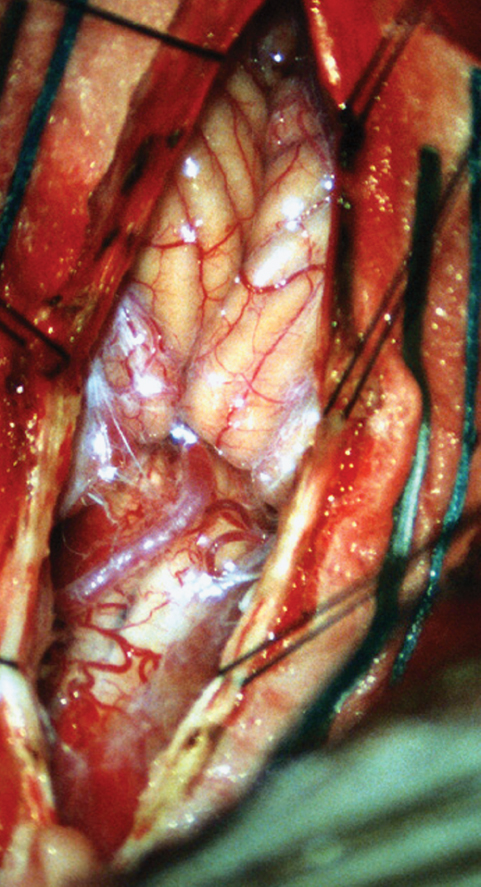
Fig. 6.7 Intraoperative photograph shows arachnoid adhesions and bands around the tonsillar ectopia. A low-lying posterior inferior cerebellar artery is also seen overlying the spinal cord.
In another study, the authors adopted a dura-splitting decompression for pediatric Chiari I malformation.73 The early clinical results were good, and this technique significantly reduced resource use.73
Others have described foramen magnum decompression with removal of the outer layer of the dura as a treatment option for syringomyelia occurring with Chiari I malformation.75,77
Recently, Sindou and Gimbert78 described decompression for Chiari type I malformation by extreme lateral foramen magnum opening and expansile duraplasty with arachnoid preservation. They claim that this type of craniocervical decompression achieved the best results with minimal complications and side effects.
Chiari II Malformations
This is a difficult entity for decision making as well as for operative detail. There is no uniform consensus on the management of these lesions. The problem lies in choosing the appropriate patients for surgery and the type and extent of the surgical intervention. In patients presenting with symptomatic Chiari II malformation due to medullary dysfunction, early surgical intervention may be life sustaining.79
Ventriculoperitoneal Shunting
According to Tubbs et al,79 a properly functioning ventricular shunt can obviate the need for hindbrain decompression. The difficulty lies with the evaluation of shunt function in these patients. In the presence of a shunt, progressive hydrosyringomyelia occurs due to shunt malfunction, unless proven otherwise.79 Periodic evaluations for brainstem malfunction need to be performed, especially by otolaryngologists and internists focusing on respiratory function, swallowing, and speech.
Posterior Fossa Decompression
In cases where progressive brainstem compression is clinically evident, posterior fossa decompression is indicated. One needs to keep in mind that the foramen magnum is wide in these patients and the torcular and the transverse sinus are low lying. Extensive bone removal may not be necessary and is dangerous if the sinuses are entered inadvertently.79 Patients may need postoperative cervical spine evaluation, because there is a possibility of delayed cervical instability.80 After midline durotomy, the arachnoid adhesions need to be lysed, and cerebellar vermis is released from compressing the cervicomedullary junction. Some patients require coagulation of excessive vermis that might be obstructing CSF flow. The foramen Magendie is carefully dissected free of the adhesions, and the CSF pathways are opened up.
Outcome
Posterior fossa decompression relieves the symptoms of syringomyelia in more than 75% of the patients and is preferred over syrinx shunting.81 If the syrinx persists and the patient is symptomatic, then a syringosubarachnoid, syringopleural, or syringoperitoneal shunt could be considered.79 Intrauterine repair of a myelomeningocele may reduce the extent of hindbrain abnormalities.82 The intrauterine repair of a myelomeningocele might reduce the fluid loss and thus the hindbrain herniation through the foramen magnum.
Chiari III Malformations
Much of the evidence comes from a relatively small number of series with very few cases. According to a recently published literature review, there are only 34 cases with Chiari III malformation.57 These patients may require VP shunting for hydrocephalus, repair of an encephalocele, and management of other congenital anomalies. If there is a hydrocephalus, VP shunting should be performed first.57 Snyder et al56 managed a patient with Chiari III malformation with an initial VP shunt. The shunt allowed the brainstem and the cerebellum to regress into the cervical spinal canal. A delayed closure of the cervical encephalocele was performed at 30 months of age. In another study, hydrocephalus was noted in seven of eight patients with Chiari III malformation, and five of them with initial hydrocephalus underwent shunting followed by closure of the encephalocele.57
The repair of the malformation follows the same principles that are applicable to open neural tube defects. The amount of neural tissue in the encephalocele varies. Great care is required to preserve neurologic function. MRI enables the surgeon to identify the amount of neural tissue in the encephalocele and the position of the brainstem and venous anomalies.50 Delayed treatment of hydrocephalus, initial severity of neurologic deficits, the amount of brain tissue within the excised encephalocele, the presence of intermittent apnea, and surgery in the newborn period were established as prognostic factors for a poor outcome.57
References
1. Carmel PW, Markesbery WR. Early descriptions of the Arnold-Chiari malformation. The contribution of John Cleland. J Neurosurg 1972; 37:543–547 PubMed
2. Loukas M, Noordeh N, Shoja MM, Pugh J, Oakes WJ, Tubbs RS. Hans Chiari (1851-1916). Childs Nerv Syst 2008;24:407–409 PubMed
3. Schijman E. History, anatomic forms, and pathogenesis of Chiari I malformations. Childs Nerv Syst 2004;20:323–328 PubMed
4. Steinmetz MP, Benzel EC. Surgical Management of Chiari Malformation. Neurosurg Q 2003;13:105–112
5. Aboulezz AO, Sartor K, Geyer CA, Gado MH. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: a quantitative approach with MR imaging. J Comput Assist Tomogr 1985;9:1033–1036 PubMed
6. Barkovich AJ, Wippold FJ, Sherman JL, Citrin CM. Significance of cerebellar tonsillar position on MR. AJNR Am J Neuroradiol 1986;7:795–799 PubMed
7. Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999;44:1005–1017 PubMed
8. Daniel PM, Strich SJ. Some observations on the congenital deformity of the central nervous system known as the Arnold-Chiari malformation. J Neuropathol Exp Neurol 1958;17:255–266 PubMed
9. Marin-Padilla M, Marin-Padilla TM. Morphogenesis of experimentally induced Arnold—Chiari malformation. J Neurol Sci 1981;50:29–55 PubMed
10. Sarnat HB. Regional ependymal upregulation of vimentin in Chiari II malformation, aqueductal stenosis, and hydromyelia. Pediatr Dev Pathol 2004;7:48–60 PubMed
11. Nishikawa M, Sakamoto H, Hakuba A, Nakanishi N, Inoue Y. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg 1997;86:40–47 PubMed
12. Gardner WJ. Hydrodynamic factors in Dandy-Walker and Arnold-Chiari malformations. Childs Brain 1977;3:200–212 PubMed
13. McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci 1989;15:1–12 PubMed
14. Padget DH. Development of so-called dysraphism; with embryologic evidence of clinical Arnold-Chiari and Dandy-Walker malformations. Johns Hopkins Med J 1972;130:127–165 PubMed
15. Gardner E, O’Rahilly R, Prolo D. The Dandy-Walker and Arnold-Chiari malformations. Clinical, developmental, and teratological considerations. Arch Neurol 1975;32:393–407 PubMed
16. Caviness VS. The Chiari malformations of the posterior fossa and their relation to hydrocephalus. Dev Med Child Neurol 1976;18:103–116 PubMed
17. Masters CL. Pathogenesis of the Arnold-Chiari malformation: the significance of hydrocephalus and aqueduct stenosis. J Neuropathol Exp Neurol 1978;37:56–74 PubMed
18. Jennings MT, Clarren SK, Kokich VG, Alvord EC Jr. Neuroanatomic examination of spina bifida aperta and the Arnold-Chiari malformation in a 130-day human fetus. J Neurol Sci 1982;54:325–338 PubMed
19. Penfield W, Coburn DF. Arnold-Chiari malformation and its operative treatment. Arch Neurol Psychiatry 1938;40:328–336
20. Goldstein F, Kepes JJ. The role of traction in the development of the Arnold-Chiari malformation. An experimental study. J Neuropathol Exp Neurol 1966;25:654–666 PubMed
21. Sarnat HB. Molecular genetic classification of central nervous system malformations. J Child Neurol 2000;15:675–687 PubMed
22. Sarnat HB, Flores-Sarnat L. A new classification of malformations of the nervous system: an integration of morphological and molecular genetic criteria as patterns of genetic expression. Eur J Paediatr Neurol 2001;5:57–64 PubMed
23. Menkes JH, Sarnat HB, Flores-Sarnat L. Malformations of the central nervous system. In: Menkes JH, Sarnat HB, Maria BL, eds. Child Neurology, 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2006:284–366
24. Iskandar BJ, Hedlund GL, Grabb PA, Oakes WJ. The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J Neurosurg 1998;89:212–216 PubMed
25. Tubbs RS, Elton S, Grabb P, Dockery SE, Bartolucci AA, Oakes WJ. Analysis of the posterior fossa in children with the Chiari 0 malformation. Neurosurgery 2001;48:1050–1054, discussion 1054–1055 PubMed
26. Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J Neurosurg 2000;92:920–926 PubMed
27. Caetano de Barros M, Farias W, Ataíde L, Lins S. Basilar impression and Arnold-Chiari malformation. A study of 66 cases. J Neurol Neurosurg Psychiatry 1968;31:596–605 PubMed
28. Menezes AH. Chiari I malformations and hydromyelia—complications. Pediatr Neurosurg 1991-1992;17:146–154 PubMed
29. Tubbs RS, Wellons JC III, Blount JP, Grabb PA, Oakes WJ. Inclination of the odontoid process in the pediatric Chiari I malformation. J Neurosurg 2003;98(1, Suppl):43–49 PubMed
30. Menezes AH. Primary craniovertebral anomalies and the hindbrain herniation syndrome (Chiari I): data base analysis. Pediatr Neurosurg 1995;23:260–269 PubMed
31. Spillane JD, Pallis C, Jones AM. Developmental abnormalities in the region of the foramen magnum. Brain 1957;80:11–48 PubMed
32. Batzdorf U. Chiari I malformation with syringomyelia. Evaluation of surgical therapy by magnetic resonance imaging. J Neurosurg 1988;68: 726–730 PubMed
33. Park JK, Gleason PL, Madsen JR, Goumnerova LC, Scott RM. Presentation and management of Chiari I malformation in children. Pediatr Neurosurg 1997;26:190–196 PubMed
34. Pillay PK, Awad IA, Little JR, Hahn JF. Symptomatic Chiari malformation in adults: a new classification based on magnetic resonance imaging with clinical and prognostic significance. Neurosurgery 1991;28:639–645 PubMed
35. Tubbs RS, McGirt MJ, Oakes WJ. Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg 2003;99:291–296 PubMed
36. Iskandar BJ, Oaks WJ. Chiari malformations. In: Albright AL, Pollack IF, Adelson PD, eds. Principles and Practice of Pediatric Neurosurgery. New York: Thieme, 1999:165–187
37. Tubbs RS, Iskandar BJ, Bartolucci AA, Oakes WJ. A critical analysis of the Chiari 1.5 malformation. J Neurosurg 2004;101(2, Suppl):179–183 PubMed
38. Miller E, Widjaja E, Blaser S, Dennis M, Raybaud C. The old and the new: supratentorial MR findings in Chiari II malformation. Childs Nerv Syst 2008;24:563–575 PubMed
39. Vigliani MB. Luckenschadel skull: a forgotten entity. Obstet Gynecol 2008;111(2 Pt 2):562–565 PubMed
40. Naidich TP, Pudlowski RM, Naidich JB, Gornish M, Rodriguez FJ. Computed tomographic signs of the Chiari II malformation. Part I: Skull and dural partitions. Radiology 1980;134:65–71 PubMed
41. Curnes JT, Oakes WJ, Boyko OB. MR imaging of hindbrain deformity in Chiari II patients with and without symptoms of brainstem compression. AJNR Am J Neuroradiol 1989;10:293–302 PubMed
42. Callen AL, Stengel JW, Filly RA. Supratentorial abnormalities in the Chiari II malformation, II: tectal morphologic changes. J Ultrasound Med 2009;28:29–35 PubMed
43. Narayan P, Mapstone TB, Tubbs RS, Grabb PA, Frye T. Clinical significance of cervicomedullary deformity in Chiari II malformation. Pediatr Neurosurg 2001;35:140–144 PubMed
44. Rauzzino M, Oakes WJ. Chiari II malformation and syringomyelia. Neurosurg Clin N Am 1995;6:293–309 PubMed
45. Stevenson KL. Chiari Type II malformation: past, present, and future. Neurosurg Focus 2004;16:E5 PubMed
46. Heinz R, Curnes J, Friedman A, Oakes J. Exophytic syrinx, an extreme form of syringomyelia: CT, myelographic, and MR imaging features. Radiology 1992;183:243–246 PubMed
47. Ansari S, Nejat F, Yazdani S, Dadmehr M. Split cord malformation associated with myelomeningocele. J Neurosurg 2007;107(4, Suppl):281–285 PubMed
48. Kumar R, Bansal KK, Chhabra DK. Occurrence of split cord malformation in meningomyelocele: complex spina bifida. Pediatr Neurosurg 2002;36: 119–127 PubMed
49. Caldarelli M, Rea G, Cincu R, Di Rocco C. Chiari type III malformation. Childs Nerv Syst 2002;18:207–210 PubMed
50. Castillo M, Quencer RM, Dominguez R. Chiari III malformation: imaging features. AJNR Am J Neuroradiol 1992;13:107–113 PubMed
51. Novegno F, Caldarelli M, Massa A, et al. The natural history of the Chiari Type I anomaly. J Neurosurg Pediatr 2008;2:179–187 PubMed
52. Albers FW, Ingels KJ. Otoneurological manifestations in Chiari-I malformation. J Laryngol Otol 1993;107:441–443 PubMed
53. Wagner JN, Glaser M, Brandt T, Strupp M. Downbeat nystagmus: aetiology and comorbidity in 117 patients. J Neurol Neurosurg Psychiatry 2008;79:672–677 PubMed
54. Honan WP, Williams B. Sensory loss in syringomyelia: not necessarily dissociated. J R Soc Med 1993;86:519–520 PubMed
55. Greenlee JD, Donovan KA, Hasan DM, Menezes AH. Chiari I malformation in the very young child: the spectrum of presentations and experience in 31 children under age 6 years. Pediatrics 2002;110:1212–1219 PubMed
56. Snyder WE Jr, Luerssen TG, Boaz JC, Kalsbeck JE. Chiari III malformation treated with CSF diversion and delayed surgical closure. Pediatr Neurosurg 1998;29:117–120 PubMed
57. I ik N, Elmaci I, Silav G, Celik M, Kalelioğlu M. Chiari malformation type III and results of surgery: a clinical study: report of eight surgically treated cases and review of the literature. Pediatr Neurosurg 2009; 45:19–28 PubMed
ik N, Elmaci I, Silav G, Celik M, Kalelioğlu M. Chiari malformation type III and results of surgery: a clinical study: report of eight surgically treated cases and review of the literature. Pediatr Neurosurg 2009; 45:19–28 PubMed
58. McGirt MJ, Nimjee SM, Fuchs HE, George TM. Relationship of cine phasecontrast magnetic resonance imaging with outcome after decompression for Chiari I malformations. Neurosurgery 2006;59:140–146, discussion 140–146 PubMed
59. McGirt MJ, Atiba A, Attenello FJ, et al. Correlation of hindbrain CSF flow and outcome after surgical decompression for Chiari I malformation. Childs Nerv Syst 2008;24:833–840 PubMed
60. Armonda RA, Citrin CM, Foley KT, Ellenbogen RG. Quantitative cinemode magnetic resonance imaging of Chiari I malformations: an analysis of cerebrospinal fluid dynamics. Neurosurgery 1994;35:214–223, discussion 223–224 PubMed
61. Zamel K, Galloway G, Kosnik EJ, Raslan M, Adeli A. Intraoperative neurophysiologic monitoring in 80 patients with Chiari I malformation: role of duraplasty. J Clin Neurophysiol 2009;26:70–75 PubMed
62. Anderson RC, Emerson RG, Dowling KC, Feldstein NA. Improvement in brainstem auditory evoked potentials after suboccipital decompression in patients with Chiari I malformations. J Neurosurg 2003;98:459–464 PubMed
63. Haroun RI, Guarnieri M, Meadow JJ, Kraut M, Carson BS. Current opinions for the treatment of syringomyelia and Chiari malformations: survey of the Pediatric Section of the American Association of Neurological Surgeons. Pediatr Neurosurg 2000;33:311–317 PubMed
64. Hayhurst C, Osman-Farah J, Das K, Mallucci C. Initial management of hydrocephalus associated with Chiari malformation Type I-syringomyelia complex via endoscopic third ventriculostomy: an outcome analysis. J Neurosurg 2008;108:1211–1214 PubMed
65. Dolar MT, Haughton VM, Iskandar BJ, Quigley M. Effect of craniocervical decompression on peak CSF velocities in symptomatic patients with Chiari I malformation. AJNR Am J Neuroradiol 2004;25:142–145 PubMed
66. Sahuquillo J, Rubio E, Poca MA, Rovira A, Rodriguez-Baeza A, Cervera C. Posterior fossa reconstruction: a surgical technique for the treatment of Chiari I malformation and Chiari I/syringomyelia complex—preliminary results and magnetic resonance imaging quantitative assessment of hindbrain migration. Neurosurgery 1994;35:874–884, discussion 884–885 PubMed
67. Klekamp J, Batzdorf U, Samii M, Bothe HW. The surgical treatment of Chiari I malformation. Acta Neurochir (Wien) 1996;138:788–801 PubMed
68. Garcìa-Uria J, Leunda G, Carrillo R, Bravo G. Syringomyelia: long-term results after posterior fossa decompression. J Neurosurg 1981;54:380–383 PubMed
69. Fischer EG. Posterior fossa decompression for Chiari I deformity, including resection of the cerebellar tonsils. Childs Nerv Syst 1995;11:625–629 PubMed
70. Dyste GN, Menezes AH. Presentation and management of pediatric Chiari malformations without myelodysplasia. Neurosurgery 1988;23: 589–597 PubMed
71. Hoffman HJ, Neill J, Crone KR, Hendrick EB, Humphreys RP. Hydrosyringomyelia and its management in childhood. Neurosurgery 1987;21: 347–351 PubMed
72. Park YS, Kim DS, Shim KW, Kim JH, Choi JU. Factors contributing improvement of syringomyelia and surgical outcome in type I Chiari malformation. Childs Nerv Syst 2009;25:453–459 PubMed
73. Limonadi FM, Selden NR. Dura-splitting decompression of the craniocervical junction: reduced operative time, hospital stay, and cost with equivalent early outcome. J Neurosurg 2004;101(2, Suppl):184–188 PubMed
74. Genitori L, Peretta P, Nurisso C, Macinante L, Mussa F. Chiari type I anomalies in children and adolescents: minimally invasive management in a series of 53 cases. Childs Nerv Syst 2000;16:707–718 PubMed
75. Isu T, Sasaki H, Takamura H, Kobayashi N. Foramen magnum decompression with removal of the outer layer of the dura as treatment for syringomyelia occurring with Chiari I malformation. Neurosurgery 1993;33:844–849, discussion 849–850 PubMed
76. James HE, Brant A. Treatment of the Chiari malformation with bone decompression without durotomy in children and young adults. Childs Nerv Syst 2002;18:202–206 PubMed
77. Chauvet D, Carpentier A, George B. Dura splitting decompression in Chiari type 1 malformation: clinical experience and radiological findings. Neurosurg Rev 2009;32:465–470 PubMed
78. Sindou M, Gimbert E. Decompression for Chiari type I-malformation (with or without syringomyelia) by extreme lateral foramen magnum opening and expansile duraplasty with arachnoid preservation: comparison with other technical modalities (Literature review). Adv Tech Stand Neurosurg 2009;34:85–110 PubMed
79. Tubbs RS, Oakes WJ. Treatment and management of the Chiari II malformation: an evidence-based review of the literature. Childs Nerv Syst 2004;20:375–381 PubMed
80. Aronson DD, Kahn RH, Canady A, Bollinger RO, Towbin R. Instability of the cervical spine after decompression in patients who have Arnold-Chiari malformation. J Bone Joint Surg Am 1991;73:898–906 PubMed
81. Milhorat TH, Johnson WD, Miller JI, Bergland RM, Hollenberg-Sher J. Surgical treatment of syringomyelia based on magnetic resonance imaging criteria. Neurosurgery 1992;31:231–244, discussion 244–245 PubMed
82. Tulipan N, Hernanz-Schulman M, Bruner JP. Reduced hindbrain herniation after intrauterine myelomeningocele repair: A report of four cases. Pediatr Neurosurg 1998;29:274–278 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








