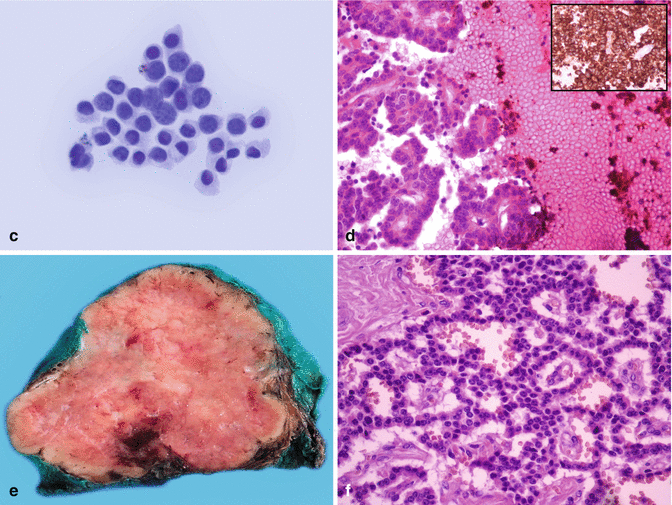
Fig. 5.1
A 35-year-old female with weight loss and jaundice. Initial investigations revealed a 7 cm mass in the pancreatic head. An endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) with rapid on-site evaluation (ROSE) was performed. (a) Ultrasound examination: solid, hard-to-puncture 7 × 7 cm lesion (arrow showing the needle in position during aspiration) (Courtesy of Dr. Christina Nichita, Gastroenterology and Hepatology, Lausanne University Hospital, Switzerland). (b) ROSE of the lesion sampled: monotonous medium-sized cells with moderate and delicate cytoplasm and dark nuclei and one pseudorosette suggestive of a neuroendocrine lesion (picture taken with a smartphone device during ROSE, Diff-Quik stain). The same slide was restained (Papanicolaou) for better final assessment, and the rest material remaining in the needle was rinsed in preservative solution for liquid-based preparations (LB). (c) LB-Papanicolaou-stained slide: bland appearing group of cells, some with larger salt and pepper nuclei, others with small amount of cytoplasm. (d) Following the initial ROSE diagnosis, two dedicated passes were performed for cell block preparation (hematoxylin and eosin stain) and immunocytochemical stains (inset, chromogranin A immunostain). The final diagnosis was neoplastic lesion, consistent with a low-grade neuroendocrine tumor. (e) Tumor resection specimen: a 4 × 5 cm mass, corresponding to the ultrasonographic image (a). (f) The histological examination confirmed the diagnosis of a neuroendocrine pancreatic tumor, NET G2
5.3 Cytomorphological Features of PanNETs
At low-power view, one can observe a clean or hemorrhagic background. In the wide spectrum of tumors with neuroendocrine differentiation, clear-cut tumor necrosis is always associated with malignant behavior. FNA material is highly cellular and contains predominantly isolated cells. Sometimes, cohesive groups show some hints of neuroendocrine architecture (ribbons and rosettes). PanNETs are highly vascular; it is thus not uncommon to find palisaded tumor cells polarized toward a capillary lumen (Table 5.1).
Table 5.1
Main cytomorphological features of PanNETs
Clean or hemorrhagic background |
Highly cellular smear with a predominance of isolated cells |
Some cohesive groups sometimes with a typical neuroendocrine architecture (rosettes, ribbons) |
Small- to medium-sized cells with plasmacytoid appearance |
Nuclei round to oval with “salt and pepper” chromatin |
Cytoplasmic changes (e.g., clearing) not to be confused with primary or metastatic carcinoma |
A cystic lesion does not rule out a PanNET! |
Around 15 % of PanNETs are partly or entirely cystic and can be confused with other cystic neoplasms. However, cytomorphology is specific of PanNETs and remains identical in cystic and solid forms. Cyst fluid can be misleading because of important variations of macro- and microscopic aspects and should be interpreted carefully. As expected, CEA and amylase levels are usually low in these lesions.
At high-power view, cells are of variable size (usually not large) and show an eccentric nucleus and enough typically granular cytoplasm to exhibit a plasmacytoid appearance. High nucleocytoplasmic ratio and nuclear molding are not characteristic of PanNETs: if found, they should prompt alternate diagnoses in the category of small-blue-round-cell tumors, small cell carcinoma being at the top of the differential. Nuclei are round to oval and regular in size. Nuclear pleomorphism, if any, does not correlate with biological behavior. Chromatin is usually described as salt and pepper, and nucleoli of various sizes can be appreciated. Cytoplasmic changes define uncommon morphological variants of PanNETs (lipid rich, clear cell, oncocytic, etc.) that should be kept in mind to avoid a wrong diagnosis of primary or metastatic carcinoma [9, 18, 19].
Features associated with malignancy include spindling or multinucleation of tumor cells, high mitotic count, and necrotic background. Histo-/cytologically proven metastasis remains the most reliable and indisputable proof of malignant behavior (Figs. 5.2, 5.3a–d, and 5.4a, b).
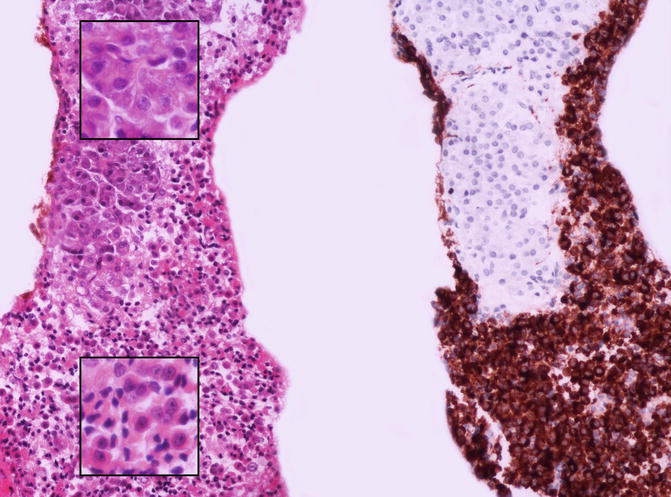
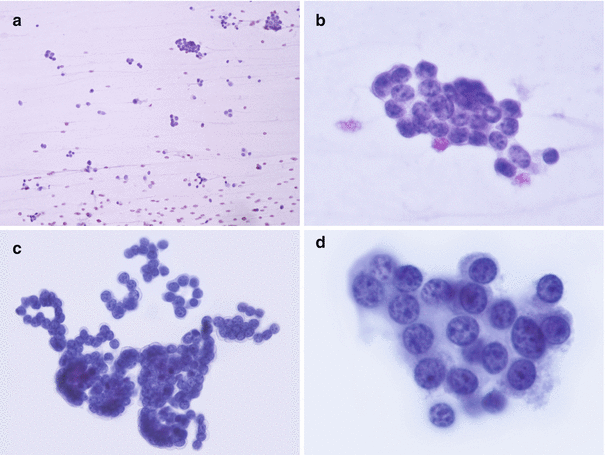
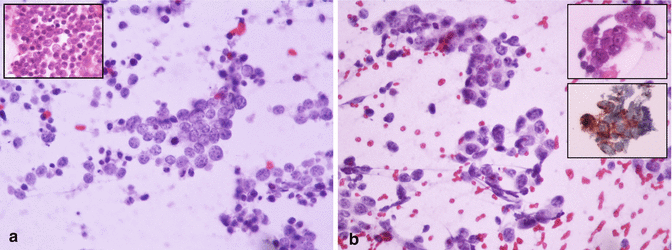

Fig. 5.2
The cell block preparation is a fundamental complement to assist in the final diagnosis as it allows to perform immunohistochemistry. In this case normal exocrine acinar cells (upper part of the picture) were intimately admixed with neoplastic cells (lower part of the picture) that stained positive for chromogranin A. The insets show at higher magnification the morphological aspect of medium-sized acinar cells (upper inset), with abundant eosinophilic cytoplasm, and small- to medium-sized neuroendocrine-looking cells (lower inset) with obvious nucleoli (left picture, hematoxylin and eosin stain; right picture, chromogranin A stain)

Fig. 5.3
Morphological aspects of well-differentiated neuroendocrine neoplasms (smear, Papanicolaou stains). (a) At low magnification the background is slightly hemorrhagic, without necrosis or inflammation. Moderate cellularity is seen with a predominantly discohesive pattern. (b) Higher magnification shows a group of cohesive cells with crowded nuclei and dark chromatin, without particular differentiation. (c) Other regions of the same slide show trabecular and rosette-like structures. This architecture, a plasmacytoid aspect of some cells, the high nucleocytoplasmic ratio, and the finely granular chromatin suggest a neuroendocrine differentiation. (d) Nuclear details: round- to oval-shaped nuclei with regular nuclear membrane contour and nucleoli

Fig. 5.4
Morphological aspects of poorly differentiated neuroendocrine carcinomas (smear, Papanicolaou stains). Crowded smears with high cellularity and background cell debris (necrosis). (a) Small-sized and loosely cohesive cells showing obvious nuclear molding, with salt and pepper chromatin and apoptotic debris. The cell block preparation (inset, hematoxylin and eosin stain) shows the same aspect. The final diagnosis was consistent with a small cell neuroendocrine carcinoma, NEC G3. (b) Larger cells with salt and pepper chromatin and macronucleoli. The cell block preparation showed only a small group of neoplastic cells (inset, upper part) that were intensely and diffusely positive for chromogranin A (inset, lower part). The final diagnosis was consistent with large cell neuroendocrine carcinoma, NEC G3
5.4 Classification/Grading
Grading PanNET is based on proliferation rate. A note of caution should be addressed on grading PanNETs on cytological specimens. The sampling could not be representative of the whole specimen, and local variations occur in these lesions; thus, higher-grade zone could be insufficiently represented in the scoring system. Proliferation index evaluated by Ki-67 on cytological material obtained from EUS-FNA procedures has shown good results, and this is either with alcohol-fixed or paraffin-embedded tumors. Moreover, high concordances have been found between cytological and histological grading to distinguish G1-G2 PanNET from G3 PanNEC (pancreatic neuroendocrine carcinoma), by counting a minimum of 100 neoplastic cells [20–22]. The grading obtained by Ki-67 on cytological material is more accurate than mitotic count, as mitotic count is not always possible on cytological samples. As an alternative to Ki-67 proliferative index, phosphohistone-H3 (PHH3) has proved to be a valid surrogate of proliferation assessment and can be paired with a computer for automated count [23].
5.5 Cytological Reporting Systems
The new nomenclature established by the Papanicolaou Society of Cytopathology applies also for PanNET, and we strongly recommend its use [4]. Briefly, the new terminology is based on a six-tiered diagnostic category system (Table 5.2).
Table 5.2




The new proposed classification systems for reporting pancreatobiliary cytology
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







