The heterogeneous tumors collected in this chapter have been lumped together rather than split into more biologically meaningful groups, because that is how clinicians present them to the pathologist. The extra-axial “category” of tumors originates in radiology: current scans usually accurately distinguish tumors arising within brain or spinal cord parenchyma (intra-axial) from those arising outside it (extra-axial). Our job as pathologists is to further subcategorize these tumors by their biological behavior.
Extra-axial tumors grow in one of two places: either outside or inside the dura. Generally, extra-axial neoplasms growing inside the dura are benign or low-grade, whereas those growing outside the dura are malignant. These rules are not absolute: meningiomas can grow through and beyond the dura and metastatic prostate cancer has an affinity for the pachymeninges of the dura. Among the extra-axial intradural tumors, meningiomas and schwannomas make up the overwhelming number of cases; others represent just a small minority of tumors reaching the pathologist. These include the uncommon solitary fibrous tumor and the rarely resected neurofibroma. Outside the dura, metastatic carcinomas and direct extensions from primary carcinomas are most common; however, other tumors also occur in this region, including various sarcomas and chordomas. This chapter will primarily discuss meningiomas but will also discuss schwannomas. Smears of metastatic tumors and chordomas will be reviewed in subsequent chapters. The other, mostly rare tumors, are not further discussed in this book.
Meningiomas
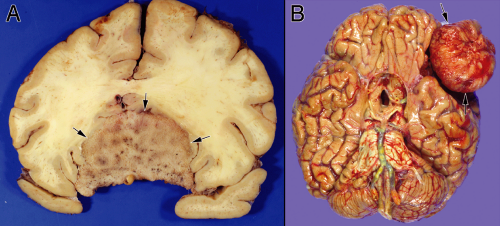
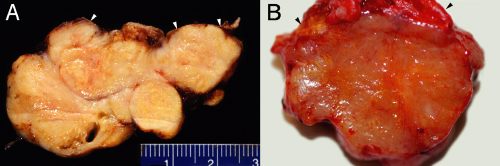
Meningiomas have the dualistic distinction of being both boring and interesting. Some can be diagnosed in the wink of an eye. Others mimic many tumor types and require more extended study to identify and predict their behavior. These tumors have a well-known female predominance, in part due to their frequent expression of estrogen receptors. Many are asymptomatic; they can be found either fortuitously or at autopsy. Given the proliferation of brain scans for weak medical reasons and the high incidence of meningiomas in the normal population, many clinically silent tumors are now removed “because they are there” rather than because they are symptomatic. Most of these neoplasms grow very slowly. The brain has an amazing capacity to accommodate such slowly growing masses. As our brains age and atrophy, the slowly increasing space within our calvarium can accommodate ever-larger meningiomas. In the elderly, tumors arising in “noneloquent” areas occasionally become huge before clinically manifesting (Figure 10-1).
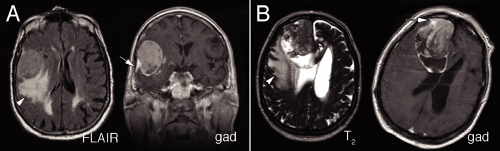
Radiologists usually accurately diagnose meningiomas; the tumors are dural-based, have a similar T1-signal intensity as brain, and enhance. These tumors arise along the entire neural axis, including within the spinal canal and posterior fossa. In the spinal canal, the tumors have a 10:1 female predominance. During development, the choroid plexus arises as an invagination of arachnoid, vessels, and ependyma; occasional meningiomas derive from these internalized arachnoid cells and arise within the ventricular space. The classic meningioma, proliferating from the dural-based arachnoid cells, shows a “dural tail” on neuroimaging (Figure 10-2). Like snow on a windowpane, a thickened dural tail forms a concave extension onto the bulging meningioma. Such tails sometimes are only visible in one of the three standard neuroimaging planes. Intact specimens display the same dural tails as the imaging. These tumors arise on the interior surface of the dura. Like normal arachnoid cap cells, the tumor cells infiltrate the dense connective tissue of the dura and firmly adhere to its surface. Intra-axial masses, such as a
metastasis to the cortex, form an acute or sharp angle with the overlying dura, rather than the more obtuse curve of a meningioma. Metastases invade into dura rather than arise from it.
metastasis to the cortex, form an acute or sharp angle with the overlying dura, rather than the more obtuse curve of a meningioma. Metastases invade into dura rather than arise from it.
Many meningiomas have minimal impact on the brain. However, more aggressive or “atypical” meningiomas show increasing effects on their underlying brain parenchyma. The brain can adapt to a slowly growing tumor by gradually indenting, leaving the underlying tissue intact. In more rapidly growing tumors, the tissue fails to compensate. Aggressive tumors typically show mass effect, pushing brain from one region to another (Figure 10-3). In response to such injury, the underlying brain becomes edematous, which manifests as increased T2 or FLAIR signal. Finally, these tumors occasionally undergo internal necrosis, which produces a heterogeneous enhancement pattern on imaging. In many cases, the tumors will retain their dural tails.
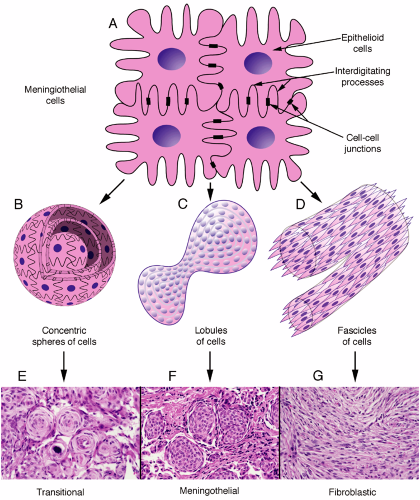
Meningiomas grow in several different patterns. However, their building-block cells are strong, adhere to each other, and have interdigitating processes. Ultrastructurally, they contain abundant intermediate filaments, have desmosomes linking their membranes, and have wildly interleaved processes (Figure 10-4A). These features become important in understanding the cytology of their smears. On a larger scale, meningiomas commonly grow in several major patterns: as small whorls of cells (Figure 10-4B), in larger lobules akin to a syncytium (Figure 10-4C), or as fascicles of spindle cells (Figure 10-4D). Tumors can breed true, showing a single architecture, but more typically display several patterns. Cross-sections of these three-dimensional structures yields the common transitional (Figure 10-4E), meningothelial (Figure 10-4F), and fibroblastic patterns (Figure 10-4G).
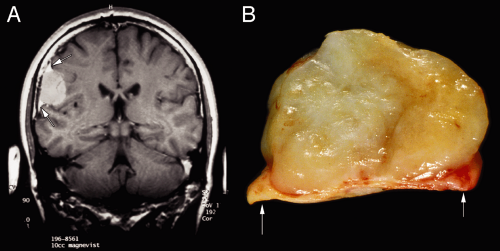
On macroscopic examination, meningiomas often have a lobular, whorled architecture, similar to uterine fibroids (Figures 10-2 and 10-5). During the crush stage of the smear, many meningiomas feel rubbery or tough. Preparing a good smear of such tumors requires increased pressure to break them apart. Others meningiomas are surprisingly soft and smear well. Even with adequate pressure, some tumors leave a thick clump at one end of the smear. If this is too bulky to coverslip, remove it with forceps and add it back to the original specimen.
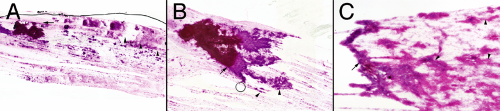
To the unaided eye (put the slide up to the light), most meningiomas form clumps and cords of cells (Figure 10-6). Such clumps reflect the strong intercellular cohesion of these neoplasms. However, unlike other cohesive tumors (e.g., schwannomas), meningiomas invariably shed at least a few helpful cells or thinner clusters of cells (Figure 10-6, A and B, arrowheads). It is this chaff arising off the main clumps that is most fruitful for further examination. How these small groups interconnect and form whorls are the most distinguishing features of meningiomas. A low-power view also reveals the cohesiveness and penchant for these tumors to form thick bridges between clusters (Figure 10-6C, arrowheads).
Although generally thick areas of a smear yield little useful cytological information, they can give large-scale structural information about a tumor. In meningiomas, look for meningothelial lobules and the thick cellular bridges connecting them (Figure 10-7). These structural units remain firmly attached to their brethren, which produces globs of cells heaped on other globs of cells. The physical
shearing of these components leaves thick bridges of cells between them.
shearing of these components leaves thick bridges of cells between them.
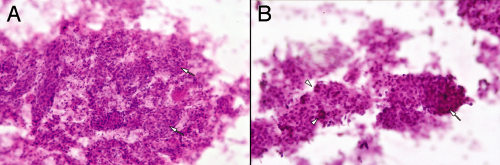
At an intermediate magnification, meningiomas characteristically reveal their abundant cytoplasm, nuclear monotony, and connections between and among the cells (Figure 10-8). In the grade I tumors, nuclei are generally slightly oval and show minimal size variation. Occasional large or bizarre cells are often degenerative or “ancient” features, rather than anaplastic indicators. These intermediate microscopic powers are best to assess the variation in population of nuclei. The eye now resolves nuclear size and shape but also compares many nuclei in the same microscopic field. At the edges of the thicker regions, smearing rips tumor cells from the larger structures. Because the tumors typically have strong intercellular attachments, this ripping pulls the cytoplasm into broad sheets or processes (Figure 10-8




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree