Fig. 11.1
Histologic features of VIPoma: (a) trabecular pattern, (b) diffuse pattern interrupted by cystic spaces containing pale serum-like material, (c) oncocytic area of a VIPoma with some lipid-rich foam cells
11.4.3 Immunohistochemical Findings
In 28 pancreatic tumors studied by one of the authors up to 1988 [19] and in three cases examined in the subsequent 24 years, immunohistochemical stainings showed positivity for the following markers of epithelial neuroendocrine cells: neuron-specific enolase (95 % of cases), synaptophysin (93 %), chromogranin A (83 %), cytokeratin (CK) AE1/AE3 (61 %), CK 8–18 (100 %), and CK19 (100 %) (Fig. 11.2). In some cases, low molecular weight CK is expressed as paranuclear dots (Fig. 11.3). Neural markers such as neurofilament protein, microtubule-associated protein (MAP2), and S-100 protein have never been detected. In contrast, neuroendocrine peptide VIP (90 % of the cases), PHM (60 %), growth hormone-releasing hormone (GHRH; 50 %), PP (53 %), alpha chain of human chorionic gonadotropin (alpha-hCG; 50 %), insulin (17 %), ghrelin (20 %), neurotensin (18 %), glucagon (10 %), and met-enkephalin (8 %) were detected. In the majority of pancreatic VIPomas, only a modest number of VIP-immunoreactive cells were present (Fig. 11.2), in agreement with the sparsely granulated ultrastructural pattern of the majority of tumor cells (Fig. 11.4) [11]. Both findings point to a defective storage mechanism with rapid release of the synthesized hormone into the blood. Production of VIP and PHM, which are normally present in neuroectodermal cells [20], may be tentatively explained by the increased plasticity in differentiation shown by the tumor cells. The frequent occurrence of PP cells in pancreatic VIPomas and the expression of both PP and VIP in the same neoplastic cells favor the interpretation that a cell line somewhat related to dorsal pancreas PP cells might be involved in the histogenesis of VIPomas [19]. Eighty percent of pancreatic VIPomas that we examined expressed the lineage marker PDX1 (Fig. 11.2), a transcription factor involved in pancreatic differentiation, which is also represented in the majority of functioning and nonfunctioning pancreatic neuroendocrine tumors [21]. Eighty percent of VIPomas show strong membranous labeling for somatostatin receptor 2A (Fig. 11.2).
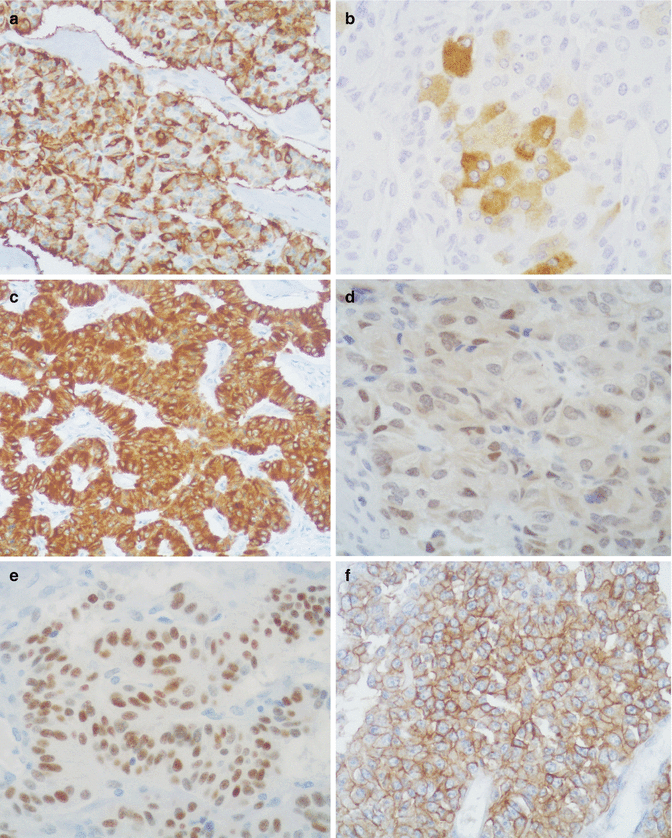
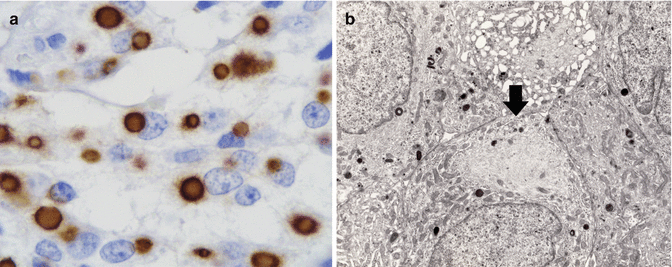
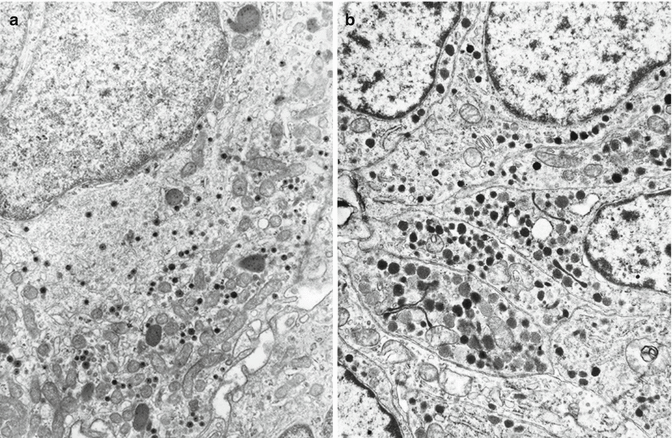

Fig. 11.2
Immunohistochemical immunoreactions of VIPomas: (a) chromogranin A, (b) VIP, (c) cytokeratin 8/18, (d) PDX1, (e) progesterone receptor, (f) somatostatin receptor 2A

Fig. 11.3
Fibrous bodies in VIPoma: (a) CK8/18 immunoreactivity; (b) electron microscopic image showing balls of cytokeratin filaments (arrow)

Fig. 11.4
Electron microscopy of pancreatic VIPoma. (a) Tumor cells containing small thin-haloed secretory granules. (b) Tumor cells containing F-type granules similar to those of PP cells
The Ki67 proliferation index of VIPomas has rarely been reported. The average Ki67 index of six of our cases and of three reported cases [22–24] was 3.99 % (range 0.55–7 %) with five tumors having a Ki67 index around 2 %, corresponding to grade 1 (G1) and four tumors having a Ki67 index more than 3 % corresponding to grade 2 of the 2010 WHO classification [25].
11.4.4 Electron Microscopy
Most tumors are formed by sparsely granulated or agranular cells with a well-developed endoplasmic reticulum and Golgi complex. Secretory granules are generally round and small (120–190 nm). Two types of secretory granules can be indentified: (1) small (120–160 nm), thin-haloed granules containing a moderately dense core, which react with VIP antibodies in electron immunocytochemical tests and (2) slightly larger (140–190 nm), in part irregularly shaped, more solid granules which react with anti-PP antibodies and reproduce the characteristics of those of PP cells of PP-rich islets of the human normal pancreas (Fig. 11.4) [26].
11.4.5 Somatic Genetics
Frequent chromosomal gains and losses involving several chromosomes have been detected in eight out of nine VIPomas examined with comparative genomic hybridization (CGH). Similar loci were involved in both VIPomas and different types of malignant neuroendocrine neoplasms. Nevertheless, a higher frequency of corresponding allelic imbalances was detected in VIPomas with LOH analysis [27–29]. In genome and gene expression analysis of a pancreatic VIPoma metastatic to the liver, defects in mismatch repair gene MSH2 and strong overexpression of the chemokine CXCR4, involved in metastasis development, have been reported [30]. Mutations of the MEN1 gene have been detected in four of nine sporadic VIPomas investigated [31, 32].
11.5 Prognosis
Most VIPomas have been classified as well-differentiated endocrine tumors (WDETs) or well-differentiated endocrine carcinomas (WDECs) according to the 2000 WHO classification [33] or as grade 1 (G1) and G2 neuroendocrine tumors (NETs) according to the 2010 WHO criteria [25]. High-grade poorly differentiated endocrine carcinomas (PDECs, WHO 2000) or neuroendocrine carcinomas (NECs, WHO 2010) have rarely been reported in association with the WDHA syndrome [34]. The average rate of metastases for patients with pancreatic VIPomas is 56.4 % [7]. Metastases are more frequently detected in the liver than in lymph nodes [7, 11, 35]. The staging systems for VIPomas are those proposed by the European Neuroendocrine Tumor Society (ENETS) [36] and by the 2010 WHO [25]. According to both systems, about one half of the cases of pancreatic VIPomas present at stage IV.
In a statistical evaluation of 179 cases of pancreatic VIPomas, Soga and Yakuwa reported 59.6 % 5-year survival for patients with metastases and 94.4 % for patients without metastasis [7]. Similar survival rates were found in our review of pancreatic VIPomas reported in the literature from 1999 to 2013 with 100 % 5-year survival for patients without metastases and 59 % for patients with metastases (Fig. 11.5).
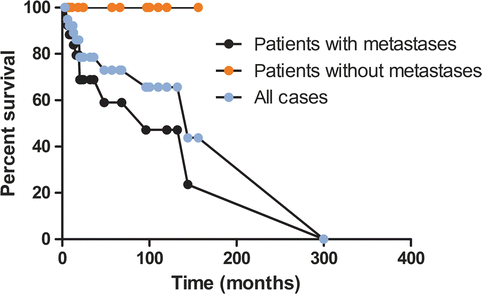

Fig. 11.5
Treatment outcome in patients with pancreatic VIPomas, reported in the literature from 1999 to September 2014, as calculated by the Kaplan–Meyer methods
References
1.
Solcia E, Capella C, Kloppel G (1997) Tumors of the pancreas, 3rd edn. Armed Forces Institute of Pathology, Washington, DC
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







