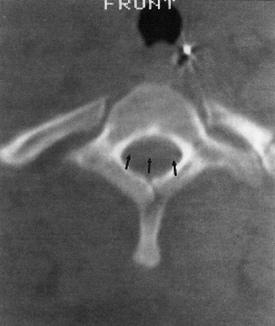
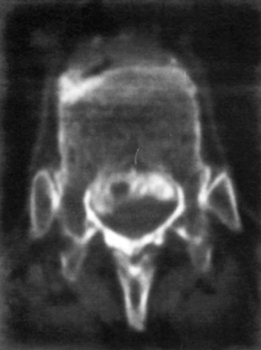
23 Intraspinal Hemorrhage Meryl A. Severson and Kenneth A. Follett Intraspinal hemorrhage resulting in spinal cord or cauda equina compression can be a true neurosurgical emergency. Rapid diagnosis and management are critical to optimize patient recovery in the setting of acute hemorrhage. Subacute and chronic hemorrhages also require timely evaluation and treatment to improve outcomes and prevent potentially devastating rehemorrhage. Intraspinal hemorrhages are epidural, subdural, subarachnoid, or intramedullary in location.1 All are managed in a similar manner and will be considered jointly. The etiology of intraspinal hemorrhage is multifactorial and classified as idiopathic, termed spontaneous in some literature,2 or secondary. All hemorrhages are spontaneous, however, and this term should be avoided when describing idiopathic hemorrhages. Causes of hemorrhage can generally be identified with careful scrutiny, but 40% to 50% have an unknown etiology3,4 and are idiopathic. Idiopathic hemorrhages may be related to structural or physiological abnormalities that aren’t recognized and may actually be secondary in nature (e.g., a vascular anomaly that obliterates itself in the process of bleeding). Secondary hemorrhages are associated with identifiable causes such as coagulopathies, vascular malformations, trauma, and iatrogenesis. Evidence for venous versus arterial origin of idiopathic spinal epidural hematomas (SEHs) has been reviewed extensively.2,5 Current opinions in the literature advocate origin in the epidural venous plexus due to the segmental nature and posterior location of these hematomas.1,6 The valveless venous system of the epidural space permits transmission of pressure waves generated in the systemic venous system (e.g., Valsalva maneuver) leading to vessel rupture1 and has been reported anecdotally.7,8 The source of idiopathic spinal subdural hematomas (SSHs) is also controversial. Valveless radiculomedullary veins cross the subarachnoid and subdural spaces.9 Rupture due to sudden increases in venous pressure10 resulting in subarachnoid hematoma that dissects to the subdural space1 has been proposed as a mechanism of formation. Secondary hemorrhages are most often related to the use of anticoagulation medications, including thrombolytics.1,11–14 As many as 30% to 50% of intraspinal hemorrhages are attributed to anticoagulant administration.4 Disease processes such as hemophilia, blood dyscrasias, or systemic lupus erythematosus (SLE) may also cause coagulopathy and are associated with intraspinal hemorrhage.15–17 Major spinal column trauma is an unusual cause of hematoma formation.18 When present, these hematomas are typically epidural and may occur in the absence of other abnormalities.19 Intraspinal hemorrhage associated with trauma may be only a single component of a patient’s injuries, may not be responsible for neurological deficits, and should be evaluated in the context of other injuries. Minor trauma such as heavy lifting and chiropractic manipulation have also been implicated in SEH formation.20,21 Vascular malformations, the most common of which are arteriovenous malformations (AVMs),22 cause 4.0% to 6.5% of intraspinal hemorrhages.3,6 Other vascular malformations associated with intraspinal hemorrhage include cavernous angiomas,22 arteriovenous (AV) fistulas,23 true aneurysms,24 and pseudoaneurysms.25 Pregnancy,26 hypertension,3 inflammatory disorders (ankylosing spondylitis, rheumatoid arthritis),4 SLE,17,27 Behçet’s syndrome,28 polyangiitis,29 pseudoxanthoma elasticum,30 cocaine use,31 exercise,7,32 and coarctation of the aorta33 have all been described in association with intraspinal hemorrhage. Bleeding may also occur from intraspinal tumors, including ependymoma,34 neurofibroma, schwannoma,35 meningioma, metastatic tumor,36 astrocytoma, and sarcoma. Vertebral body abnormalities such as hemangioma37 and Paget’s disease38 may also lead to SEH formation. Iatrogenic causes include spinal surgery,13 ventriculoperitoneal shunting,39 neuraxial anesthesia,13 and lumbar puncture (LP).11,15 Hemorrhage following LP may be subarachnoid,40 subdural,11,15 or epidural.40 The risk of intraspinal hemorrhage following LP is increased if the tap is traumatic, if anticoagulation is started earlier than 1 hour post-LP, or if the patient is on aspirin therapy.41 Silverman et al42 found no instances of hemorrhage in hemophiliacs given factor replacement prior to LP. Intraspinal hematomas occur in individuals of any age, from in utero43 to the elderly.10 Approximately half of affected individuals are over the age of 50.3 Bleeding is most commonly epidural and typically localized to the dorsal or dorsolateral portion of the canal.44 Males are slightly more often affected by epidural bleeding (~1.5:1)44 and females by subdural bleeding (~2:1).9 Subdural hemorrhage most often affects thoracic and thoracolumbar regions,11 epidural hematoma the cervicothoracic or thoracic region,1,6 subarachnoid hematoma the thoracic spine,1 and intramedullary hemorrhage the thoracic spinal cord.1 Pain is usually the first symptom of intraspinal hemorrhage followed by signs and symptoms of neural element compression.1,2,32 Patients may present with subacute or chronic pain symptoms,1 or even with a remitting/relapsing course.45 Signs of neurological dysfunction generally evolve over the span of hours but progression may be very rapid. Deficits typically include sensory loss with paraparesis or paraplegia, urinary retention, cauda equina syndrome, and even priapism.1,19,35 Patients may present with Brown-Séquard,14 central cord,46 or anterior cord syndrome.47 The differential diagnosis includes a variety of causes of acute spinal cord dysfunction, including disk herniation, spinal fracture (pathological or traumatic), infection (e.g., epidural abscess), transverse myelitis, infarction, tumor, trauma, or dissecting abdominal aortic aneurysm. The history and physical provide a foundation for establishing a diagnosis, but radiographic evaluation is required for definitive diagnosis. Myelography, computed tomography (CT), and magnetic resonance imaging (MRI), the preferred initial modality, have been used in the evaluation of these patients. Myelography was the procedure of choice prior to the advent of MRI. It is still useful, especially in conjunction with postmyelogram CT scanning, for patients who cannot undergo MRI scanning (e.g., those individuals with non-MRI-compatible implanted medical devices or metal). Myelography is contraindicated in patients with coagulopathy and necessitates a delay of evaluation while coagulation parameters are checked and possibly corrected. The LP for myelography may be “dry” or technically difficult in the presence of clot.9 CT is noninvasive and may be used in patients with coagulopathy and those with contraindications to MRI scanning. It may be especially useful in cases involving spinal column bone pathology by virtue of its sensitivity in detecting bone abnormalities (e.g., fractures and osteolytic or -blastic changes). Its sensitivity in demonstrating hematoma is limited, however. Blood appears hyperdense acutely, whereas subacute and chronic hemorrhages appear isodense (Fig. 23-1). The sensitivity and specificity of CT scanning are improved with intrathecal contrast (Fig. 23-2); however, LP is contraindicated in those patients with coagulopathy. MRI with gradient echo is the preferred study1 and often the only one required. It is noninvasive, generally well tolerated, and very sensitive and specific for identifying the presence and extent of intraspinal hemorrhage. MRI may also reveal the underlying cause of secondary hemorrhages. The MRI appearance of hematomas varies with the age of the hematoma. On T1- and T2-weighted images, respectively, blood is iso-/hypointense and hyperintense hyperacutely; iso-/hypointense and hypointense acutely; hyperintense and hypointense early subacutely; hyperintense and hyperintense late subacutely (Fig. 23-3 and Fig. 23-4); and iso-/hypointense and hypointense chronically.48 SSHs are concave, whereas SEHs are convex masses on sagittal imaging10 (Fig. 23-5 and Fig. 23-6). Gadolinium may help in identification of structural lesions such as tumors, infections, or slow-flow vascular malformations. The dural sac may enhance due to hyperemia in the subacute stages after a hemorrhage, providing better demarcation between the thecal sac and hematoma.49 Figure 23-1 Unenhanced axial computed tomographic (CT) scan through the upper thoracic spine reveals a slightly hyperdense mass occupying the ventral half of the canal (posterior aspect delineated by arrows), consistent with acute epidural hematoma. Even when hyperdense, hematomas can be difficult to distinguish from surrounding structures on CT imaging.
Etiology
Presentation
Evaluation
Intraspinal Hemorrhage
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








