and Robert E. Schmidt2
(1)
Sunnybrook and St Michael’s Hospitals, University of Toronto, Toronto, ON, Canada
(2)
Division of Neuropathology Department of Pathology, Washington University School of Medicine, St. Louis, MO, USA
Leprosy is the most common cause of neuropathy in the underdeveloped world (Misch et al. 2010). Notwithstanding a spectacular decrease in global prevalence since 1982, leprosy consistently remains a public health problem in 32 countries, mostly in Africa, Asia, and South America. The detection rate (figures from 2010) for leprosy is about 250,000 new cases being registered each year. The rare cases originating in North America are confined to certain regions in Louisiana, Texas, California, and Hawaii. With present immigration patterns in North America, clinicians can expect to see more patients with this disease, with the Philippines, Southeast Asia (Vietnam, Cambodia, and Laos), South America, and the Caribbean being the particularly high-risk regions of origin. In North America secondarily transmitted cases are exceedingly rare. Exceptionally, the disease is identified in patients who seem to have no reason to be so afflicted (Mastro et al. 1992). In the St. Michael’s Hospital nerve biopsy experience, leprosy has been the fifth most common specific diagnosis, with the patients all emigrating from endemic areas in Southeast Asia or India.
Unless an individual is identified as being at high risk for leprosy, the diagnosis of leprous neuropathy depends on histological examination of the skin or nerve. The diagnosis is usually easily made by skin biopsy when cutaneous lesions are present. However, nerve biopsy is essential for the diagnosis of primary neuritic leprosy, which exhibits no skin lesions (vide infra). The identification of Mycobacterium leprae (ML) in a sample can be obtained by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) of the heat shock 65 gene (hsp65), which is ML specific (Martiniuk et al. 2007).
Mycobacterium leprae is a fastidious, acid-fast, Gram-positive, slightly curved bacillus measuring 1–8 μm in length and 0.2–0.5 μm in diameter (Carpenter and Miller 1964). Functionally the bacterium is nonmotile and aerobic and cannot form spores. It is an obligate intracellular parasite and survival is unfavorable outside the host cell. Reproduction of the organism occurs by binary fission at approximately 10–14 day intervals assuming optimal growth conditions of 27–30° Celsius (by comparison, doubling time for virulent strain of M. tuberculosis is about 20 h). The only confirmed methods of interindividual disease transmission and spread occur by aerosol droplets expelled through the upper airway mucosa and shedding of bacilli from skin lesions by infected patients having the lepromatous form of the disease, the organisms then entering contacts by the nasal mucosa (Ridley 1988; WHO 1988). The occurrence of acid-fast bacilli (AFB) in the skin (Figueredo and Desai 1949; Chatterjee et al. 1976) and nasal mucosa of apparently healthy subjects (Chacko et al. 1979) has been reported. ML can survive outside the human body for 45 days; this raises the possibility of indirect transmission of the bacillus from soil contamination (Lavania et al. 2008; Turankar et al. 2012). The local development of skin leprosy following the inoculation of bacilli from a contaminated tattoo needle is rare, but not exceptional (Ghorpade 2002). Only about 5 % of individuals exposed to ML will progress to an infected state (Newell 1966). The incubation period is between 2 months and 10 years (Ridley 1988). Vulnerability to ML appears to be selective, and there is evidence of an inherited component to this susceptibility (Shields et al. 1987; Misch et al. 2010). The circular genome sequence of M. leprae (TN strain, Tamil Nadu, India) was unraveled in 2001 by Cole’s research group (Eiglmeier et al. 2001; Monot et al. 2005). Recent epidemiologic studies in Colombia have shown that multiple different strains of ML vary in distribution from Andean and Atlantic locales (Cardona-Castro et al. 2013).
While human beings are the principal reservoir of the infection, in the Americas the armadillo provides a reservoir. Consequently, in the latter part of the twentieth century, the nine-banded armadillo was introduced as an experimental animal model for human leprosy. The proportion of human cases of leprosy attributable to the armadillo remains unknown, but some are well documented (Hamilton et al. 2008; Sharma et al. 2013). Studies are in progress in the estimation that two-thirds of acquired human cases in southern USA have armadillo-derived ML in the lesions. In 1960, Shepard was able to reproducibly induce granulomata containing acid-fast bacilli in the footpads of mice after inoculation with bacteria harvested from nasal passages (22/22 “takes”) or biopsies (12/16“takes”) of human leprosy cases (Shepard 1960). The footpads of the athymic nude mouse are susceptible to ML infection serving as experimental models of leprosy and biological sources of abundant M. leprae (Alter et al. 2011).
In leprosy, neural involvement occurs early and invariably (Job 1989), the human Schwann cell and endoneurial endothelium having a particular affinity for the bacillus (Ridley 1988). This predilection of M. leprae for Schwann cells is likely determined by the organism selectively binding to the G domain of the laminin-alpha 2 chain, which is a unique component of the Schwann cell basal lamina (Rambukkana et al. 1997). Clinical leprosy lies in a spectrum between two extremes: tuberculoid and lepromatous according to the Ridley–Jopling classification, which is based on skin lesion type and bacterial load. At the tuberculoid pole a brisk cell-mediated immune reaction is generated against the organism, while those patients with lepromatous leprosy are anergic toward ML, have multiple lesions, and are pluribacillary. Most patients can be classified along this spectrum based on histology (vide infra). The disease does not remain static, but evolves spontaneously or in response to therapy. Workers refer to a transition toward the tuberculoid pole as upgrading and one toward the lepromatous pole as downgrading. While some researchers report spontaneous resolution of tuberculoid and indeterminate infections, spontaneous regression does not occur in lepromatous leprosy patients (Misch et al. 2010). Associated with the initiation of treatment types 1 and 2, immune-mediated reactions continue to be major complications. An upgrading of the host’s immune response can result in acute neuritis, as can formation of immune complexes to ML antigens (vide infra: acute neuritis). Two types of immune-mediated reactions are observed in leprosy that affects around 30 % of patients with multibacillary disease during and after treatment (Rodrigues and Lockwood 2011). Type 1 or reversal reactions (RR) represent the sudden activation of a Th1 inflammatory response to ML antigens. RR often occurs after the initiation of treatment in patients at the borderline or toward the lepromatous pole of the leprosy spectrum (LL, BL, BT, or borderline [BB] category) and reflects a switch from a Th2-predominant cytokine response toward a Th1-predominant cytokine response (Britton and Lockwood 2004; Scollard et al. 2006). Risk factors for RR intrinsic to the host include age (Ranque et al. 2007) and some genetic variants, although the latter have not been intensively investigated. Type 2 reaction erythema nodosum leprosum (ENL) is an acute systemic inflammatory condition involving tumor necrosis factor (TNF), tissue infiltration by CD4+ cells (Kahawita and Lockwood 2008), and deposition of immune complexes and complement (Britton and Lockwood 2004). ENL also occurs in LL or BL patients and is more commonly seen in patients with a high bacterial index (multibacillary disease). The host factors that regulate the immunoclinical phenotypes of ENL and RR are poorly understood (Sapkota et al. 2010).
12.1 Clinical Manifestations
There are no sensitive serological tests to routinely detect leprosy cases resulting from ML infection. Consequently, the current diagnosis relies on clinical observations combined with invasive procedures to confirm acid-fast bacilli in slit skin smears or immunopathological changes in biopsies of skin lesions or nerve for determining the presence of the disease and its characterization (Geluk 2013). Although the presence in sera of IgM antibodies against phenolic glycolipid-I (PGL-I) is positive in nearly all leprosy patients with high bacillary loads, most paucibacillary leprosy patients do not develop detectable antibodies against PGL-I. Furthermore, almost 50 % of individuals with positive anti-PGL-I IgM responses never develop leprosy and many of those who develop leprosy do not have PGL-I antibodies. Thus, the detection of asymptomatic M. leprae infection, allegedly a principal source of infection, remains elusive. The clinical hallmarks of leprosy are sensory loss and skin lesions. The specific manifestations depend on the host’s ability to react against the organism. Reviews are provided by Sabin and co-workers (1993), Pearson and Ross (1975), and, most recently, de Freitas and Said (2013).
12.1.1 Lepromatous Leprosy
In lepromatous leprosy cell-mediated immunity to the organism is absent, bacteria disseminate hematogenously throughout the tissues. Microscopic examination of affected regions reveals a striking number of organisms located within a variety of cells, often with a minimal local inflammatory response. Disease progression occurs slowly and insidiously, with late and symmetrical neuropathic manifestations. Because M. leprae proliferates more effectively in a cool environment, nerves in certain characteristic areas are affected early: the pinnae, dorsum of the hand, elbow, dorsum of the foot, and anterolateral leg. This pattern may mimic a stocking-glove peripheral neuropathy, but the palms, soles, and skin between the digits remain unaffected. The initial manifestations are sensory because sensory nerves run a more superficial course, but as the disease progresses it affects mixed nerves, with the ulnar nerve at the elbow being particularly vulnerable. Infiltration with massive numbers of bacteria and inflammatory cells, along with fibroblast proliferation (Tzourio et al. 1992), results in fusiform enlargement of superficial nerves, typically including the greater auricular, ulnar, and common peroneal. Reflexes are spared until the late stages, because the reflex arc involves deep nerves.
12.1.2 Tuberculoid Leprosy
In pure tuberculoid leprosy, the host’s cell-mediated immune response is relatively well preserved and features a Th1 T-cell cytokine response, vigorous T-cell reaction to M. leprae antigen, and containment of the infection in well-formed granulomata (Scollard et al. 2006). Spread occurs locally along neurovascular structures (Ridley 1988). Symptomatic neuropathy develops early and is focal and asymmetric, and temperature dependence of involved sites is not prominent. The skin lesions show epithelioid cell granulomata that invade through cutaneous layers, and organisms are not detected. Small sensory nerves as well as larger mixed nerve trunks are damaged as they pass through local regions of inflammation. Thus, in addition to loss of sensation over the skin lesions, this form may present as a mononeuritis multiplex. Evolution of neurological deficits occurs more rapidly than in lepromatous leprosy. Nerve enlargement (Fig. 12.1a–c) takes place as a result not of massive infiltration with bacteria as in the lepromatous form, but of the exuberant inflammatory response.
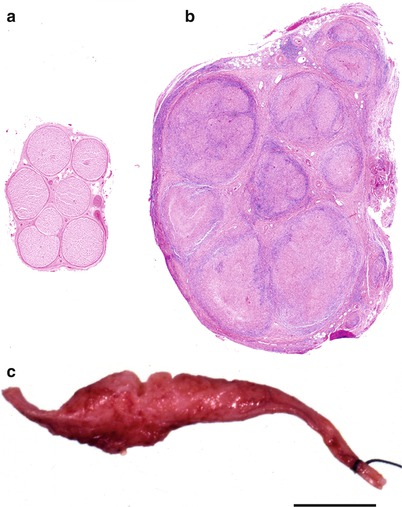

Fig. 12.1
Cross sections of sural nerve. Normal sural nerve (a) and tuberculoid leprosy (b). Note the massive enlargement of the nerve and presence of multiple granulomata with effacement of the microscopic anatomy. (c) Gross specimen of tuberculoid leprosy. (a, b: paraffin, H&E, 20×; c, surgical specimen, bar = 1 cm)
12.1.3 Borderline Leprosy
Borderline (BB, dimorphous) leprosy takes the middle ground between tuberculoid (TT) and lepromatous (LL), with intermediate clinical and pathological features. The lesions can show substantial numbers of organisms as well as a histiocytic infiltrate. Classification as borderline tuberculoid (BT) or borderline lepromatous (BL) depends on which side of the spectrum dominates. Clinically, the nerve involvement tends to favor low-temperature areas as in the lepromatous form, but brisk local reaction in these areas produces more severe nerve damage. If the host immune response is insufficient to prevent hematogenous dissemination, and a cell-mediated response still exists, a particularly devastating neuropathy may result.
12.1.4 Pure Neuritic Leprosy
In leprosy, damage to nerves can occur before, during, and after treatment and can result in disability and long-term disfigurement (Rodrigues and Lockwood 2011). A cohort study in Ethiopia showed that 47 % of 594 new cases already had established nerve function impairment at the time of diagnosis (Saunderson 2000). Pure neuritic leprosy refers to involvement of peripheral nerves in the absence of skin lesions and was seen in 4.3 % of 11,000 patients with leprous neuropathy in one series (Osuntokun 1980). The clinical picture does not predict the histological subtype (vide infra). Nerve involvement may range from a small superficial nerve twig with isolated skin patch of anesthesia to infection of several nerves. Widespread neuropathies may ensue with greater areas of sensory deficits, sometimes preceded by pruritus, paresthesia, and rarely pain. The nerves more likely to be involved are the superficial mixed nerves trunks of upper and lower extremities. This includes the ulnar nerve at the elbow, the median nerve above the carpal tunnel, the radial nerve at the spiral groove, the radial cutaneous nerve in the lower forearm, the peroneal nerve above the fibula head, the tibial nerve above the ankle, and the facial nerve at the region of the zygomatic bone (Ooi and Srinivasan 2004). The sensory nerves, such as the superficial peroneal, posterior auricular, and sural nerves, may also be involved.
12.1.5 Acute Neuritis
Although leprous neuropathy is usually chronic, leprosy reactions can cause an acute neuritis. In an upgrading reaction the host’s immune status improves, and areas where the organism previously lay dormant become damaged by a cell-mediated inflammatory response. An acute neuritis is also seen with erythema nodosum leprosum, believed to be due to immune complex formation and deposition, particularly at sites where large numbers of organisms are located. Both types of acute neuritis tend to occur within the first year after initiation of therapy, but may develop spontaneously (Ridley 1988).
12.1.6 Treatment of Leprosy
It is important to establish histological type before initiating pharmacotherapy. The WHO recommends multidrug therapy with rifampicin and dapsone for paucibacillary disease and with rifampicin, dapsone, and clofazimine for patients with multibacillary leprosy. These regimens will effectively eradicate M. leprae in most patients (Rodrigues and Lockwood 2011). Vaccination with BCG protects some people from developing leprosy.
12.2 Pathology
12.2.1 General Considerations
The histological classification of leprosy is largely based on the examination of skin lesions. Ridley’s text (1988) provides an excellent review. Of note is that the skin and nerve histology are often incongruous (Ridley and Ridley 1986). Generally, the viable bacterial load is higher in the nerve, probably because it is a relatively protected site: Organisms within Schwann cells tend not to incite an inflammatory response, and neural architecture hinders the influx of lymphocytes (Pearson and Ross 1975; Ridley 1988). For example, Nilsen et al. (1989) found that 8 of 11 patients with multibacillary leprosy in the nerve had paucibacillary leprosy in the skin. This observation might give rise to concern regarding the usefulness of skin biopsy in characterizing the nature of the disease and providing a rational approach to treatment. However, Ridley and Ridley (1986) point out that such data simply demonstrates the “protected site” behavior of the leprosy organism, that skin responses are more indicative of the general tissue response, and that consequently skin biopsy is still the best guide for classification and treatment of the patient.
Falsely negative histology in nerve biopsy for leprous neuropathy probably occurs (Jacob and Mathai 1988), but the frequency is unknown. Nerve biopsy is likely more sensitive than skin biopsy (Nilsen et al. 1989). To minimize the incidence of false negatives, it is best to biopsy clinically involved nerves rather than proceed with blind sural nerve biopsy. Some investigators use the index branch of the radial cutaneous nerve (Antia et al. 1975) and others use the radial cutaneous nerve itself (Nilsen et al. 1989). A biopsy report indicating the absence of bacilli implies that the specimen has been embedded in toto and that appropriately stained step sections through the entire tissue block have been examined.
Even when the diagnosis of leprosy is strongly suspected, atypical neuropathy can justify a nerve biopsy. In a study of primary neuritic leprosy from an endemic area (Jacob and Mathai 1988), only 38 of 77 patients with neuropathy had leprosy histologically; nineteen of 54 clinically enlarged nerves demonstrated no evidence of leprosy. False negatives were undoubtedly present, but a number of alternative diagnoses, including polyarteritis nodosa, hereditary neuropathy, and inflammatory demyelinating neuropathy, were made. These results suggest that in certain situations there may be a need for histological confirmation of the diagnosis even in endemic areas. Moreover, there are cases when the disease appears to be inactive based on examination of skin scrapings and subcutaneous nerves, yet the patient develops new neurological deficits. Nerve biopsy may show that the neural component of the disease is still active (Enna et al. 1970; Liu and Qiu 1984; Srinivasan et al. 1982), again reflecting the potential dissociation between cutaneous and neural disease activity. Leprous neuropathy was a surprise diagnosis on two of our surgical intraoperative consultation sections for peripheral nerve tumor.
12.2.2 Lepromatous Leprosy
12.2.2.1 Light Microscopy
In lepromatous leprosy, there is an uneven involvement of fascicles with relative preservation of the overall architecture (Fig. 12.2). Macrophages and Schwann cells filled with organisms and debris (foamy cells) appear in the epineurium, endoneurium, and perineurium, accompanied by a strikingly mild local reaction (Fig. 12.3a). In the perineurium, foamy macrophages infiltrate and separate individual layers, fibroblasts and perineurial cells proliferate, and collagen is deposited, producing a striking “onion skinning” of the nerve fascicles (arrow, Fig. 12.3b). Perineurial cells often demonstrate swelling and foamy changes and the bacillary macrophages predominating. Lymphocytes are not numerous in polar lepromatous (LL) leprosy, but may occur in subpolar forms. Perivascular cuffing is common but never reaches the level of true vasculitis, except in acute reactions (vide infra).
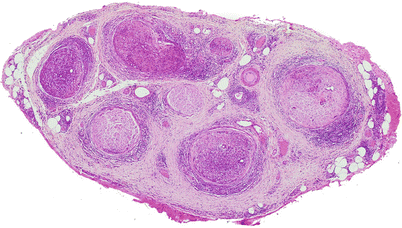
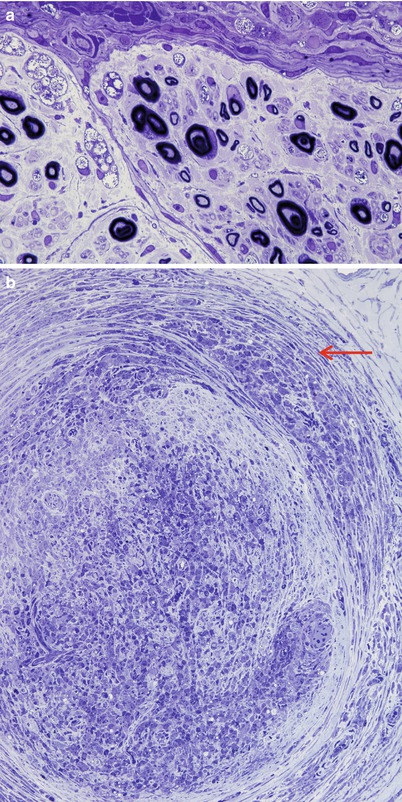

Fig. 12.2
Lepromatous leprosy. While the fascicular arrangement is preserved, there is prominent inflammation in the epineurium, perineurium, and endoneurium. Some fascicles are more involved than others. (Paraffin, HPS, 27×)

Fig. 12.3
Lepromatous leprosy. (a) Heavy intracellular bacterial load exists with no overt inflammatory response. (b) Note separation of perineurial leaves by inflammation (arrow) and heavy infiltration of the endoneurium by mononuclear cells. (1 μ toluidine blue-stained plastic sections; magnification, a, 1,000×; b)
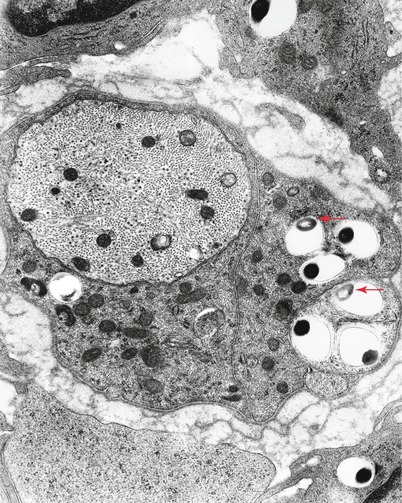
Bacilli abound (Fig. 12.4a) and are found within macrophages (“lepra cells”) as intracellular “globi” containing dozens or even hundreds of bacteria as seen in H&E-stained nerve (Fig. 12.4b) and plastic sections (Fig. 12.4c). Even in heavily involved nerves, some fascicles may be relatively spared (arrow, Fig. 12.4d). Toluidine blue (in plastic resin-embedded sections) will demonstrate M. leprae in Schwann cells, perineurial cells, fibroblasts, endothelium, and infrequently axons (Fig. 12.5a–c). Fite (Fig. 12.5d) or auramine rhodamine stains for acid-fast organisms often demonstrate impressive numbers of organisms. Any cell type, but most often Schwann cells or macrophages, can show this foamy appearance, representing cellular reaction to the accumulation of living and dead organisms. Macrophages often display prominent perivascular localization. Giant cells, granulomata, and massive lymphocytic infiltration are not features of lepromatous leprosy.
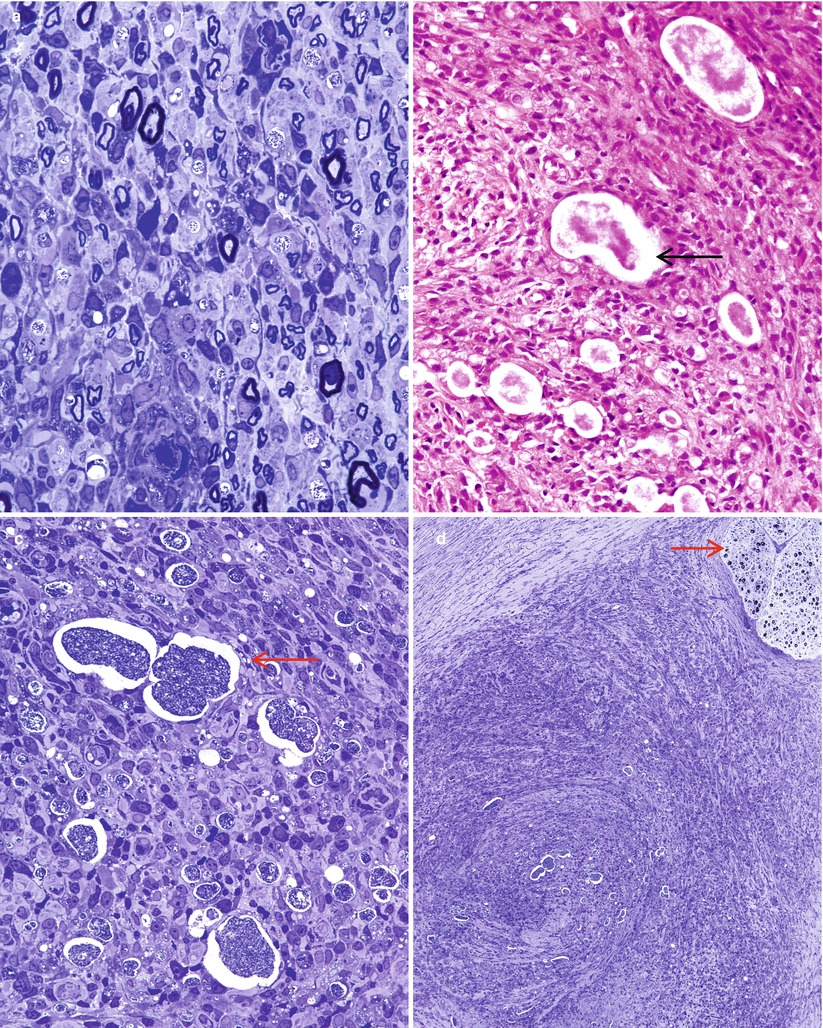
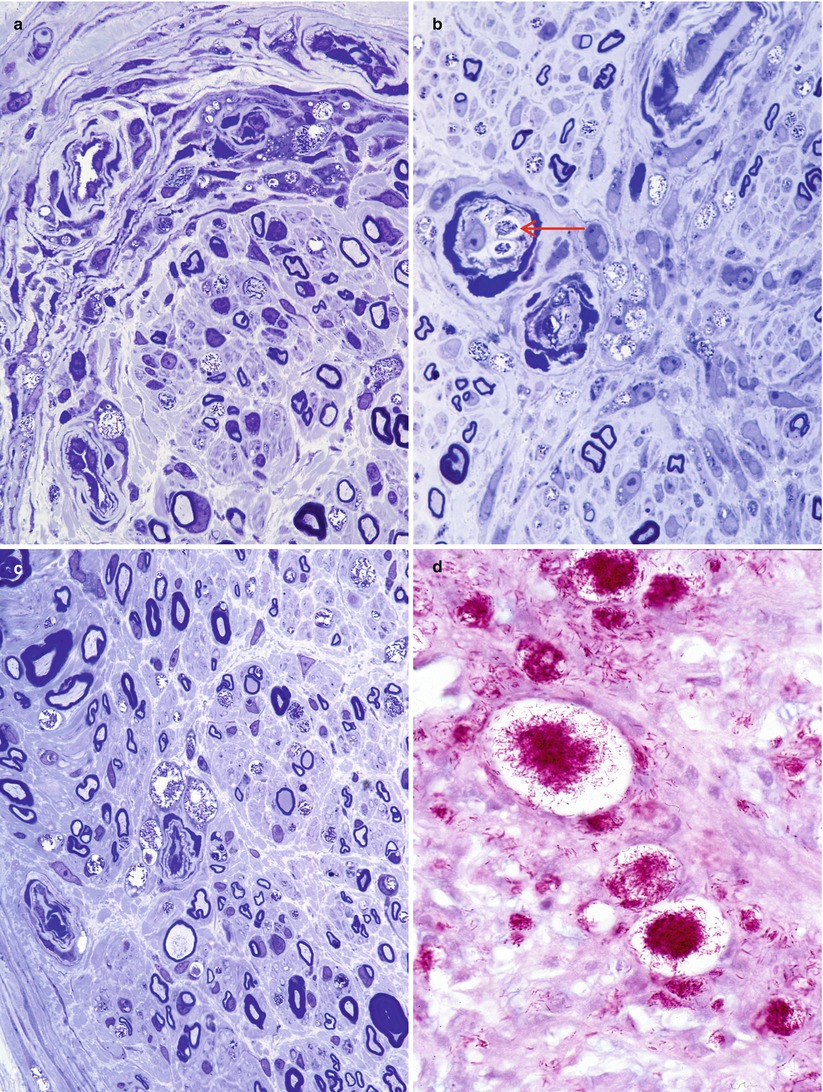

Fig. 12.4
Lepromatous leprosy. (a) Numerous organisms are noted in the endoneurium intermingled with intact MFs. (b, c) Organism-laden macrophages or globi (arrows) are seen by H&E (b) and toluidine blue stains (c), frequently without visible nuclei. The endoneurial damage here is more severe, with no recognizable myelinated axons. (d) Extensive endoneurial and epineurial inflammation largely spares an adjacent fascicle (arrow) (1 μ toluidine blue-stained plastic section; magnification, a, 400×; b, c 600×; d, 100×)

Fig. 12.5
Lepromatous leprosy. Numerous bacilli are demonstrated within perineurial (a) and endothelial (arrow, b) cells. Bacilli-laden macrophages collect adjacent to endoneurial vasculature (c). Fite stain for organisms demonstrates the extent of endoneurial bacilli (d) (a–c, 1 μ toluidine blue-stained plastic section; magnification, 1,000×; paraffin section, Fite stain, 1,000×)
Initially, despite the presence of innumerable organisms, the neural architecture and axon numbers are relatively preserved. Segmental myelin changes may predominate early on, with thinly myelinated and naked axons and even occasional onion bulbs (Job 1971). With progression of the disease, axonal degeneration becomes significant and involves both myelinated and unmyelinated fibers. Regenerating clusters may be present and can feature prominently in successfully treated patients (Jacobs et al. 1993). If untreated, most nerve fibers will ultimately degenerate.
With progression of the disease, collagen is laid down in ever increasing amounts, sometimes to the point where the nerve is entirely replaced with fibrous and hyaline material. Bacilli are often demonstrable, albeit in small numbers, even in the nerve that has been totally replaced by collagen and large vacuolated cells containing lipid and bacterial debris can be prominent long after treatment has finished (Fig. 12.6a–c).
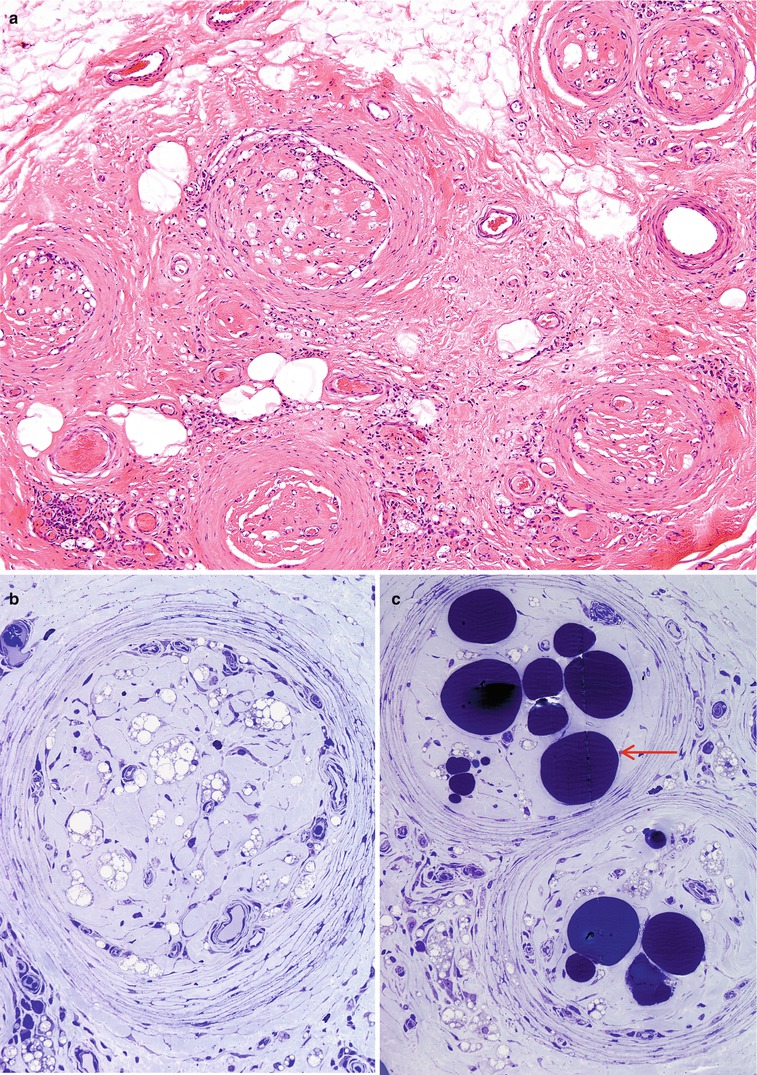

Fig. 12.6
Lepromatous leprosy, burnt out stage. No residual bacilli are present on Fite, fluorescent, or toluidine blue preparations. (a, b) The endoneurium is devoid of any nervous elements and contains foamy histiocytes and hyaline connective tissue. (c) The large osmiophilic globules (arrow) are adipocytes in the endoneurium. (a, Paraffin section, H&E, 100×; b, c, 1 μ toluidine blue-stained plastic section, 400×)
12.2.2.2 Ultrastructural Examination
Electron microscopy shows the organisms as membrane-bound round or rod-shaped electron-dense structures surrounded by a clear halo (Fig. 12.7) (possibly partially composed of bacterial metabolites and/or denatured host cytoplasmic components) (Imaeda 1965; Job 1971). Degenerating bacilli show loss of osmiophilic staining (arrow, Fig. 12.7) or irregularities in their cell wall (arrows Fig. 12.8a, b) (Rees and Valentine 1964). Bacteria are easily found in macrophages and Schwann cells of unmyelinated fibers and less frequently in Schwann cells associated with myelinated fibers. Bacteria do not appear extracellularly (Figs. 12.8, 12.9a, b, and 12.10). Initially, the Schwann cell shows no reaction, but with increasing numbers of organisms, signs of degeneration appear. Macrophages can contain large numbers of bacilli, most in a degenerating state.