Benign:
S1: Latent tumors
(G0 T0 M0)
S2: Active tumors
(G0 T0 M0)
S3: Aggressive tumors
(G0-1 T0-1 M0-1)
Malignant:
Stage I
Low grade
A: Intracompartmental
(G1 T1 M0)
Low grade
B: Extracompartmental
(G1 T2 M0)
Stage II
High grade
A: Intracompartmental
(G2 T1 M0)
High grade
B: Extracompartmental
(G2 T2 M0)
Stage III
Any Grade
Any T
(G1-2 T1-2 M1)
25.3.1.1 Benign Tumors
Benign tumors are divided into three stages. First stage tumors (S1) are “latent” tumors which are inactive and usually asymptomatic. Their feature is to be surrounded by a true capsule, expression of latency. Once identified as latent tumors, these do not need to be submitted to a treatment based on oncological principles as they tend to grow very slowly if at all. Intraosseous enchondromas, osteochondromas, hemangiomas, or lipomas (extremely rare) can be staged into this category. Palliative surgery is required for spinal cord compression or spinal instability due to pathologic fractures, while in most cases these tumors must be followed with clinical and imaging observation.
Second stage (S2) “active” benign tumors tend to grow at a certain rate albeit slowly and often become symptomatic. These are surrounded by a thin layer of fibrous capsule and induce the formation of a reactive inflammatory tissue, which may be seen on MRI (“pseudocapsule”). Oncologic treatment of S2 tumors consists of surgical excision. Embolization, cryotherapy, and radiofrequency ablation are other modalities that may be used in conjunction with surgery or alone.
Third stage (S3) benign tumors (“aggressive”) are rapidly growing tumors with a very thin or absent capsule. These tumors invade the neighboring compartments and usually have a wide reactive hypervascularized “pseudocapsule.” They usually grow to become large enough to be visible on radiographs, technetium bone scans are usually significantly positive, and CT and MRI demonstrates the aggressive nature of the tumor. Treatment consists of surgical excision of adequate aggressiveness along with the help of one or more of the surgical adjuvants delineated above.
25.3.1.2 Malignant Tumors
Malignant tumors are studied based on the concept of “grade” and classified into two groups as low grade and high grade. These are further subdivided into two categories of A and B based on the relation of the tumor with the compartment it has originated from. A is used for the tumors that are still within the compartment at the time of diagnosis, and B for those that have extended beyond that compartment or has originated at a location with no natural boundaries and therefore does not constitute a compartment (e.g., intrapelvic). Based on this, a low-grade stage IA tumor is one that remains inside the vertebra itself and by contrast, a stage IB tumor invades paravertebral compartments. These tumors usually have thick pseudocapsules of reactive tissue and small, microscopic tumor islands within that reactive zone called “satellite nodes.” The acceptable resection margin is therefore wide resection if possible. High-grade tumors are likewise divided into stages IIA and IIB. These are very rapidly growing tumors with no reactive tissue; on the other hand they do have not only satellites lesions but also a significant risk of skip metastases (foci of tumor outside the main mass, completely isolated). High-grade tumors are identifiable on plain radiographs, but MRI is needed in addition to show the entire extension of the tumor and the absence of reactive zone. Treatment is wide en bloc excision as radical excision in the spinal column is impossible [16].
25.3.2 Surgical Staging
After the definitive diagnosis and oncological staging has been established, the next step before biopsy should be surgical staging. The most widely used scheme was developed by Weinstein for primary spine tumors and later modified by Boriani and coworkers to become the WBB (Weinstein, Boriani, Biagini) system [17]. This system is useful for defining the local extent of the tumor and therefore eventually dictates the type of resection needed. Vertebra is divided into 12 radiating zones in a clockwise order (from 1 to 12), five concentric layers from paravertebral extraosseous to the intradural region (A to E) (Fig. 25.1), and the longitudinal extension of tumor as expressed by the number of involved spinal segments is added as a separate parameter. The major advantage of this system is that it delineates the relation of the lesion with the spinal cord and therefore intrinsically marks out the amenability of the tumor to wide resection. These authors recommend that in order not to endanger the spinal cord and to control the epidural space, the surgeon should aim to resect wedge sectors of the vertebra [16].
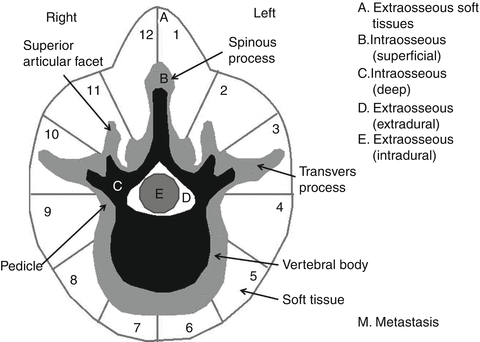

Fig. 25.1
The WBB staging system for primary spine tumors (Adapted and redrawn from the original in Ref. [6])
Another classification system is proposed by Tomita and coworkers. This system is composed of a two-part numeric system and incorporates a detonation of tumor location providing a simplified scheme for describing the extent of vertebral involvement. The first numeric part describes the affected anatomic site, comprising 1 vertebral body, 2 pedicles, 3 lamina, transverse and spinous processes, 4 spinal canal, and 5 paravertebral area. The second numeric part describes tumor extension in numbers ranges from 1 to 7. These authors tended to consider type 1, 2, and 3 lesions as intracompartmental, type 4, 5, and 6 lesions as extracompartmental, and a type 7 lesion as a multisegmental tumor or one with multiple-skip lesions [18]. For the sake of simplicity only the WBB system will be used in this text.
25.4 Biopsy
Biopsy is an essential step before planning the treatment. The purpose of this procedure is to provide the pathologist with an amount of pathological tissue both quantitatively and qualitatively representative for diagnosis. Its volume must be adequate for different staining and for immunohistochemical studies. The tissue must not be removed from necrotic, reactive, or fibrotic areas; it must be taken from the core of the vital tumor. Biopsy is by definition an intralesional procedure and therefore includes the high risk of spreading tumor cells in the surrounding tissue, with the obvious consequence of increasing the risk of local recurrence. The most important surgical principle is to include the biopsy route within the line of incision that will be used at the time of definitive surgery, particularly in case of en bloc resection, whose specimen will necessarily include the entire biopsy tract from the skin to the tumor mass. For this reason, the biopsy approach should never be performed along the anatomical extracompartmental spaces, as performed in elective non-oncologic orthopedic surgeries. The biopsy approach must always be performed inside muscles, in order to make easier the removal of the tract. Following these principles, biopsy can be performed through percutaneous or open techniques [19]. Open biopsy should be performed by the surgeon who will perform the definitive surgery; it may result in substantial blood loss and morbidity, but the surgeon can obtain a relatively large amount of tissue for diagnosis decreasing the likelihood of a sampling error. A particular care should be adopted to control bleeding and avoid hematoma, which is a severe complication of biopsy as the tumor cells can be seeded on a wide area, almost impossible to resect later on.
Percutaneous biopsy (performed by fine core needle or best by a trocar) is a relatively simple procedure and has been proven to be safe and effective when performed under CT-scan image guidance [20]. On the other hand, selection of the optimal biopsy technique depends on the differential diagnosis, the location and extension of the lesion, and the potential definitive treatment plan. Although there is the theoretical possibility of having intraoperative frozen sections, the present authors do not recommend it on a routine basis as in our hands it has been associated with a substantial rate of diagnostic errors, totally unacceptable for tumor surgery in spinal column. Therefore it should be reserved for cases whose imaging is pathognomonic (like some ABC or some OO) or for the confirmation of the adequacy of surgical margins if necessary. Open biopsy should be avoided when a malignant bone tumor is suspected on clinical and imaging studies due to the highest risk local recurrence. Transpedicular image-guided trocar biopsy allow to remove adequate sample from anterior elements without contaminating the thoracic or abdominal cavities; to reduce the risk of seeding from the empty pedicle, it can be filled with acrylic cement [16].
25.5 Surgical Treatment of Spinal Tumors
The goal of the surgery for pediatric spinal primary tumors is to allow the best local and systemic control; conversely, the treatment of metastatic and systemic diseases is mostly palliative, aiming at pain relief, decompression of neural structures, provide correct alignment, stability, and possibly mobility to the spine [21].
Tumor removal in case of metastatic and systemic disease is reasonable if the specific tumor type has low sensitivity to chemotherapy and radiation therapy.
A commonly accepted terminology for surgical procedures and for definition of tumor extent is needed for surgical planning. Lesions in the pediatric spine are often more challenging to treat than lesions of similar behavior elsewhere in the musculoskeletal system. “Curettage” describes the piecemeal removal of the tumor. As such, it is always an intralesional procedure. “En bloc” indicates an attempt to remove the whole tumor in one piece, together with a layer of healthy tissue. The term “intralesional” is appropriate if the surgeon has been within the tumor mass at any time during surgery; “marginal” is appropriate if the surgeon has dissected along the pseudocapsule, the layer of reactive tissue around the tumor; and “wide” is appropriate if ablation could be performed outside the pseudocapsule, removing the tumor with an undisturbed shell of healthy tissue. This wide en bloc procedure can be called “excision” or “resection.” “Radical resection” means the en bloc removal of the tumor and the whole compartment of tumor origin which is virtually impossible for a spine tumor because of the ring shape of the vertebral body around the neural structures and also because of the fact that some compartments such as the epidural or subdural spaces extend from sacrum to cranium [16]. Slow-growing but locally aggressive primary tumors that may be easily treated elsewhere in the skeleton may be unresectable and potentially lethal in certain locations in the spine. The surgical approach should be planned carefully and must achieve the prescribed appropriate margins. While intralesional removal may be associated with excellent outcomes for many patients with benign latent tumors and benign active tumors, more aggressive surgery is indicated for some locally aggressive benign tumors and many malignant tumors. Non-metastatic malignant tumors are ideally removed with wide surgical margins, when technically feasible.
As described above, the treatment depends on diagnosis, natural history, location, size of the lesion, as standardized in the Surgical Staging System (SSS) which dictates the type of surgical treatment to be used. Benign latent lesions (S1) do not require oncologic treatment as they are latent. Benign active tumors (S2) do have growth potential: Intralesional excision usually can be performed with a low rate of recurrence. Benign aggressive tumors (S3) infiltrate neighboring compartments and have wide reactive hypervascularized pseudocapsules. En bloc resection with wide/marginal margins is often indicated for reasonably acceptable recurrence rates when possible. If not, intralesional excision and additional local adjuvant therapies such as phenol or alcohol administration, cryotherapy with liquid nitrogen or abundant use of polymethylmethacrylate (PMMA) may be necessary. Depending on specific sensitivity, radiation therapy can be helpful as an adjuvant. The side effect of radiation therapy on growing bone must be considered, together with the risk of secondary radio-induced tumor in patients with a long life expectancy [22].
Management of malignant spinal tumors is more complex and requires a multidisciplinary approach. Early detection of the tumor followed by complete excision is advisable [18]. Again the SSS helps to delineate the progressive stages of a given tumor and the specific implications for surgical treatment and provides guidelines for the use of adjuvant therapy. Advances in chemotherapy and techniques for resection and reconstruction have expanded the role of local surgical management. For optimal surgical treatment oncological staging of lesion is essential. The grade of the tumor as a sign of the general behavior, presence of metastatic lesions, location and extension of the tumor in the spinal column are important factors. Local control of the malignant pediatric spinal tumor can only be achieved with a well-planned wide resection. However, wide en bloc excision procedure may be impossible in some cases because of the location and the extent of the tumor. Even if the spinal cord and nerve roots are sectioned above and below, the epidural space represents a compartment extending from the skull to the coccyx. Therefore, a tumor-free margin en bloc resection is not possible when a stage II malignant tumor is encroaching the canal.
Three different en bloc resection surgeries have been defined: vertebrectomy, sagittal resection, and resection of the posterior arch. Vertebrectomy implies removal of all the elements of the vertebra, which may be total, or hemi- in the sagittal plane. These procedures can be performed in staged, sequential, or simultaneous anterior and posterior approaches or in a single stage through posterior approach [18, 23–26]. Lesions involving the posterior elements of the spine are obviously submitted to en bloc excision by posterior approach [16].
Reconstruction of the spine after resection before maturity requires a further concern as long fusions can be followed by secondary impairment of sagittal balance, while short fusions – particularly if associated with muscle and ligament sacrifice as required in tumor resection – can create shortly a segmental instability [27].
25.5.1 Specific Spinal Tumors
25.5.1.1 Benign Tumors
Eosinophilic Granuloma (Langerhans Cell Histiocytosis)
It represents the Langerhans cell histiocytosis form localized only in the skeleton, different from Hand Schuller Christian disease and Letterer Siwe disease. (Table 25.2) This lesion is a reactive proliferation of Langerhans cells forming granulomas and may produce focal destruction because of this essentially inflammatory character. Clinical manifestations range from a single bony lesion to multiple granulomas in bones and soft tissues to systemic forms of disease. The incidence of spinal involvement ranges from 7 to 25 % [28–33]. It is commonly seen in children less than 10 years of age and is more common in males [34–36]. The most common presenting symptom is pain [34, 35, 37, 38]. Vertebra plana is the typical radiological appearance caused by the partial or complete collapse of the vertebral body [28]. Vertebra plana is not the onset image, it is the image of the collapse following the erosive initial activity of the EG (Fig. 25.2). The initial imaging is hard to be distinguished from a lymphoma or Ewing’s sarcoma and biopsy is mandatory. It is important to note that the collapsed vertebral body is located between two normal discs in order to differentiate from infectious disease. Asymmetrical vertebral collapse can lead to the scoliosis or severe kyphosis but the most common deformity is mild to moderate kyphosis [39] (Fig. 25.3). Neurological symptoms due to vertebral collapse may rarely be seen.
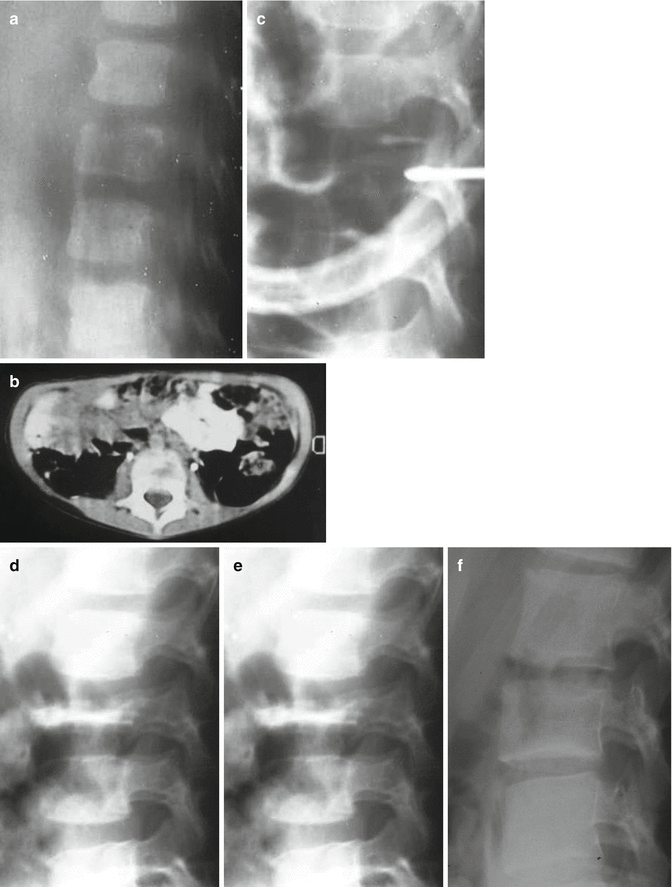
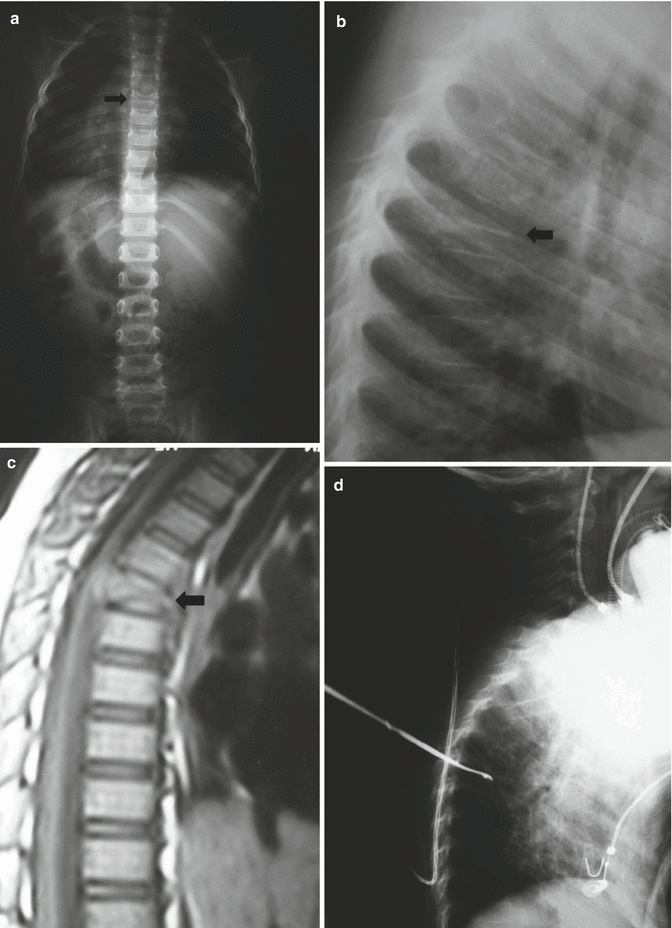
Table 25.2
Frequently encountered benign and malignant tumors of pediatric spine
Benign lesions |
Eosinophilic granuloma (Langerhans cell histiocytosis) |
Aneurysmal bone cyst |
Osteoid osteoma |
Osteoblastoma |
Osteochondroma |
Giant cell tumor |
Fibrous dysplasia |
Non-ossifying fibroma |
Malignant lesions |
Ewing’s sarcoma |
Osteosarcoma |
Leukemia |
Neuroblastoma (metastatic) |
Wilms’ tumor (metastatic) |
Teratoma (metastatic) |
Lymphoma (metastatic) |

Fig. 25.2
A 4-year-old male, back pain and muscle spasm. Case observed in 1992 (a, b) Sagittal Tomography and CT scan show initial lytic changes in L2 vertebral body. (c) Biopsy was performed by transpedicular open approach and allowed the diagnosis of Eosinophilic granuloma. (d) An orthosis was advised. The standard radiogram 2 months later shows the tyical aspect of a vertebra plana. No pain. The patient was allowed to leave the orthosis. (e) Three years later initial reconstruction is evident. (f) At 10-year follow-up the reconstruction is complete, no functional loss

Fig. 25.3
A 5-year-old male presenting with back pain. (a, b) AP and lateral plain X-rays demonstrating the typical appearance of vertebra plana (arrow). (c) T1-weighted sagittal MR image demonstrating the totally preserved disc spaces (arrow) (d) Intraoperative lateral X-ray taken during transpedicular biopsy
A skeletal survey or bone scan should be done to rule out other lesions of EG which is associated with multifocal disease. MRI is helpful for the differential diagnosis from malignancy. Histology of these lesions has three main components that are lipid-containing histiocytes with “coffee-bean” appearance, eosinophils, and Langerhans giant cells.
Solitary lesions are usually a self-limited disease. Treatment is somewhat controversial, but it is clear that many patients heal their lesions without any treatment (see Fig. 25.2) or for that matter, any treatment other than biopsy. Observation with or without spinal immobilization with cast, body jacket, orthosis, or collar that can be used for few months to several years has been the standard modality of treatment. This conservative treatment allows the load sharing of the anterior column and may produce an enhancement in the growth plate activity leading to a possible restoration of vertebral height [40]. Raab and coworkers have reported that 18.2–97 % vertebral body height restoration was possible in conservatively treated patients. It appears that the age of the patient is an important factor in this context. If the lesion had been identified at least 4 years before skeletal maturity, remaining growth capacity is usually enough for adequate remodeling regardless of location at the cervical, thoracic, or lumbar regions [37]. Radiotherapy, chemotherapy (only for disseminated form), and steroid injections have been advocated with no proven benefits over observation for solitary lesions. Operative treatment is only necessary in the rare instance such as neurological involvement secondary to vertebral collapse, compression of the spinal cord, extraosseous extension and instability of spine, or persistent pain.
Osteoid Osteoma (OO) and Osteoblastoma (OBL)
These lesions are most frequently seen in first two decades of life and show a propensity (greater for OBL) for the posterior elements of the spine. They may be located in the pedicles, transverse processes, laminae, and spinous processes. The overall rate of spine localization ranges between 10–41 % for OO and 30–50 % for OBL [23]. Pain is the predominant symptom and is usually worse at nights and activity. It may resolve with the use of nonsteroidal anti-inflammatory drugs (NSAID). Night pain and dramatic response to NSAID should evoke the clinical suspect of OO. On the other hand, OBL pain has a lower response to NSAIDs. These tumors often produce pain before they become visible on plain radiographs. A CT scan is often required to diagnose the lesions which have the typical appearance of osteosclerosis surrounding a radiolucent nidus of less than 2 cm in diameter for OO and greater than 2 cm for OBL (Figs. 25.4 and 25.5). Technetium bone scan can be useful in establishing the diagnosis while roentgenograms are still negative, and the pain definition by the patient is vague and non-localizing by showing a non-specific but intense, well-defined focal uptake of activity [41–43]. OO is typically a benign latent self-limiting lesion that has a tendency to spontaneously regress over several years whereas OBL are usually locally aggressive tumors.
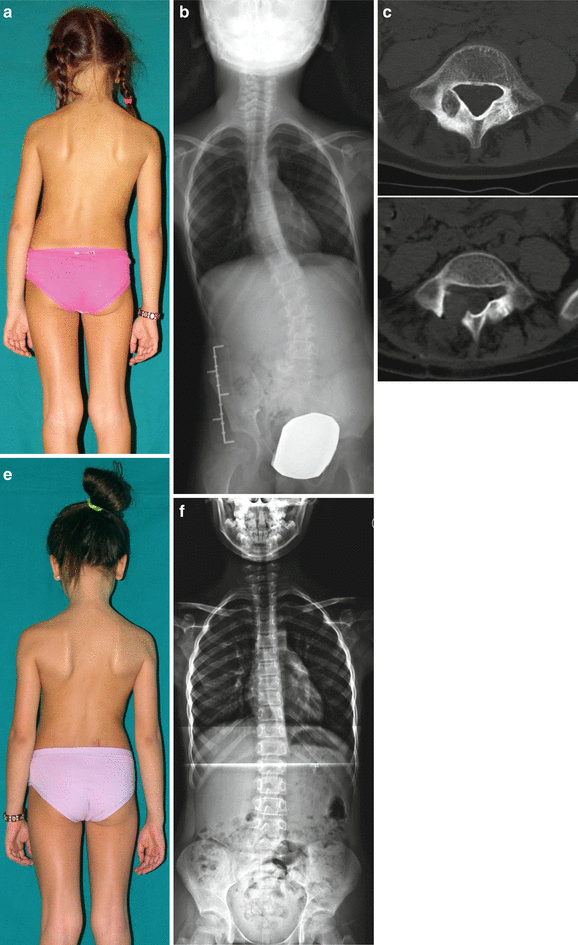
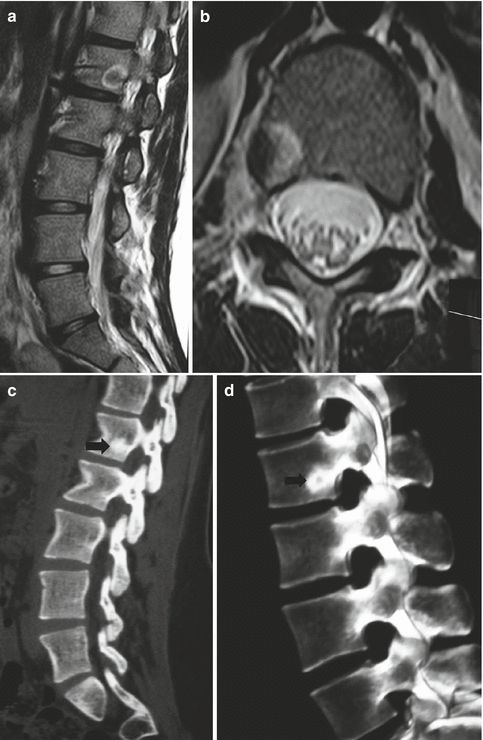

Fig. 25.4
A 6-year-old female. (a) Painful scoliosis. (b) Standard standing radiogram shows a left lumbar scoliosis with relevant torsional component. (c) CT scan shows a small lytic area surrounded by a sclerotic reactive bone, located in the right posterior arch of L5. Imaging is consistent with the diagnosis of osteoid osteoma. (d) Postoperative CT scan; complete excision of the nidus. (e) Six months later the deformity is reduced. (f) Standing radiograms showing recovering of normal alignement in the coronal and in the transverse plane

Fig. 25.5
A 14-year-old female presenting with back pain that is worse at night time and reasonably responsive to salicylates. MR imaging was prescribed, revealing a completely hypointense nucleus surrounded by a relatively hyperintense zone on sagittal (a) and axial (b) T2-weighted images. (c, d) Sagittal and 3-D reconstructions of CT images demonstrating a sclerotic zone surrounding a relatively lytic nidus typical for osteoid osteoma (arrows)
Painful scoliosis is another fairly well-recognized presentation of OO and (less frequently) OBL [6, 7, 44], the incidence of being the initial symptom of spinal OO and OBL ranges from 25 to 63 % [8, 45]. Lesions with scoliosis are more common in thoracolumbar spine than cervical spine and have been identified as the most common cause of pain provoked reactive scoliosis [46]. Saffuidin and coworkers have reported on the typical findings associated with scoliosis in OO and OBL, based on a series in which 63 % of patients had scoliosis overall. Lesions were mostly located on the concave side near the apex. Asymmetrical location of lesion within the vertebral body or neural arc appeared to be the most significant factor for the development of scoliosis whereas a lesion at the center of vertebral body (e.g., spinous process) had the least likelihood. It was postulated that asymmetrical inflammatory effect of the lesion caused asymmetrical muscle spasm and secondary scoliosis. Cervical lesions were associated with a minor chance of developing scoliosis, predominantly at the lower cervical spine, but asymmetrical inflammation may lead to torticollis [9]. Considering that congenital and idiopathic scoliosis are always painless, a painful scoliosis should always rise the suspect of OO and induce to submit the young patient to an isotope scan.
If the patient’s symptoms can be managed with NSAIDs without any significant side effects, a trial of medical treatment may be prescribed. Long-term medical treatment was found to be as effective as surgical treatment [47]. However, such prolonged use of drugs is often associated with at least gastrointestinal irritation and may lead to severe hemorrhage. Surgical treatment should be considered if medical treatment cannot be used or is not successful, that of OO being intralesional excision. There is no need for the removal of the entire sclerotic reaction; however, the nidus should be completely removed to ensure good pain relief and to prevent recurrences. As pain is usually radically improved after complete resection of the nidus, it can attest to the completeness of the excision as well. Spinal deformity improves in almost all patients within 15 months [6, 8, 23].
Contrary to OO, treatment of OBL consists of complete surgical excision. Curettage has been advocated in the past, but with an unacceptable rate of recurrence. Even marginal excisions carry a recurrence risk of about 10 % [48]. Radiotherapy has been advocated in the past because of these relatively high rates of recurrence after surgery but has been mostly abandoned now in the era of modern spinal surgery, as it may be associated with the danger of malignant transformation of these lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








