16
Middle Fossa Craniotomy
William House refined the middle fossa approach in 1961.1 Initially, he used this approach to decompress the internal auditory canal (IAC) in cases of extensive otosclerosis with sensorineural hearing loss. Although not beneficial for that condition, it quickly became evident that this approach was suitable for small acoustic tumors in patients with good hearing.2,3 The middle fossa procedure is a hearing-preservation approach.4–6 For tumors, it allows complete exposure of the lateral end of the IAC, so no blind dissection is necessary at the fundus. This is a definite advantage over the retrosigmoid approach, which does not provide exposure of the lateral third of the IAC without loss of hearing. Disadvantages include the fact that this is a technically difficult approach. Because of the superior location of the facial nerve in the canal, dissection of the tumor may subject the facial nerve to more manipulation than in other approaches. But with the advent of facial nerve monitoring, problems of the facial nerve are kept to a minimum and facial nerve outcome is no different than when using the translabyrinthine approach for tumors of similar size, at least in experienced hands.7 Retraction of the temporal lobe is required for the duration of the drilling and tumor removal, which usually does not exceed 1 to 1½ hours.
In the treatment of acoustic tumors, the translabyrinthine and middle fossa approaches are often compared with the retrosigmoid approach. The translabyrinthine approach provides wide access to the cerebellopontine angle (CPA) with little cerebellar retraction, and permits exposure of the entire facial nerve from the brainstem to the stylomastoid foramen.8,9 The exposure is mostly extradural, minimizing possible injuries to the brain and to the cerebellum. In addition, it provides a more direct and anterior perspective. A shorter distance separates the surgeon from the contents of the CPA.10,11 The translabyrinthine approach enables identification of the facial nerve at the lateral end of the IAC before tumor dissection. The main disadvantages of the translabyrinthine approach are the need to sacrifice hearing in the operated ear, the limited exposure of the lower part of the CPA, and the limited access to the neural contents of the foramen magnum and foramen jugulare.
The retrosigmoid approach provides a panoramic view of the posterior fossa from the tentorium to the foramen magnum. Access is provided to the cerebellar hemisphere, the lateral aspect of the pons and medulla, and the root entry zone and cisternal course of cranial nerves V to XI. Although exposure superiorly is limited by the tentorium, this approach could be combined with a middle fossa or transtentorial exposure. The retrosigmoid approach represents a modification of the classical suboccipital approach. Krause12 and others first employed the suboccipital route during the latter portion of the 19th century. In this procedure, a large bone flap is removed from the suboccipital area, with the anterior limit of the dissection being the first mastoid cell. Superiorly, bone is removed up to the inferior margin of the transverse sinus. The retrosigmoid approach offers a more favorable angle of view into the CPA and a markedly reduced need for cerebellar retraction than the classic suboccipital approach. It does not pose a risk of air embolism or quadriplegia, as does the classic suboccipital approach, which places the patient in the sitting position. Advantages are wide access to the CPA and the potential for hearing preservation. The retrosigmoid approach is capable of addressing most lesions of the CPA.13 Disadvantages include a higher incidence of postoperative headaches and cerebrospinal fluid (CSF) leaks when compared with the translabyrinthine approach.14–17 The lower incidence of postcraniotomy headaches in the translabyrinthine approach may be due to lesser cerebellar retraction, reduced dissection of the suboccipital musculature, and completion of all the bony work before dural opening. The higher incidence of CSF leaks in the retrosigmoid approach is explained by the difficulty in sealing all of the cells in the petrous apex, especially when it is extensively pneumatized. Another disadvantage of the retrosigmoid approach is poor exposure of the ventral aspect of the pons and medulla due to the relatively posterior angle of view. The posterior aspect of the clivus is obstructed by the course of cranial nerves V to XI.
Microsurgical resection is the traditional method of treatment for acoustic tumors. The translabyrinthine and the middle fossa approaches to the CPA are, in our opinion, the approaches of choice for removal of most acoustic tumors because they provide access to the whole length of the IAC. This chapter describes the middle fossa craniotomy, its indications, and the results.
♦ Indications
The main indication for the middle fossa craniotomy is removal of intracanalicular vestibular schwannomas in patients with good hearing. Arbitrarily, good hearing has been defined as a speech reception threshold of at least 30 dB and a word recognition score of at least 70%. Exceptions to this rule do exist, as in the case of poor hearing in the contralateral ear or in bilateral tumors. Typically we use the middle fossa approach for tumors up to 2 cm in size in patients with at least serviceable hearing.18 In selected cases, the extended middle fossa craniotomy is used. In general, this approach is reserved for patients younger than 65 years of age because of the fragility of the dura and the underlying temporal lobe in older patients. Another indication for the middle fossa craniotomy is decompression of the IAC in patients with neurofibromatosis type 2 to allow the tumor room for expansion to preserve the hearing on that side. The middle fossa craniotomy also can be used to perform vestibular neurectomies when indicated, although we prefer the retrolabyrinthine craniotomy for vestibular neurectomy.
The middle fossa approach is also used to repair symptomatic superior canal dehiscence and in patients with large tegmen defects with encephaloceles and CSF leaks.
♦ Preoperative Studies
Magnetic resonance imaging (MRI) is necessary to show the exact location and size of the tumor. Tumor size is thought to be an important prognostic factor, with some studies showing that the smaller the tumor, the better the chances at hearing preservation. MRI determines the relationship of the tumor to the brainstem, cerebellum, and IAC. Particular attention should be paid to a major vessel loop crossing within the tumor. The nature of the tumor and its consistency, cystic versus solid, can also be evaluated.
Other predictive factors are provided by audiometry, auditory brainstem response (ABR), and electronystagmography (ENG).19 The better the hearing in the ear, the better the chance of preserving useful hearing. Patients with a normal ABR have a better chance of postoperative hearing preservation. Superior vestibular nerve tumors indicated by abnormal caloric responses are also thought to be favorable prognostic factors. However, in some studies, tumor size and ENG results were not predictive.
♦ Surgical Technique
A standard endotracheal anesthesia induced with thiopental, and a short-acting muscle relaxant is used. Intravenous furosemide 40 mg and mannitol 1 g/kg body weight are administered for brain relaxation when the skin incision is made. Preoperative antibiotics are also administered. The blood pressure is monitored using an arterial line.
Facial nerve monitoring is done for the entire period of the operation. Cochlear nerve function is monitored using ABR; once the tumor is exposed, direct cochlear nerve potentials are obtained just prior to tumor removal.
The patient is placed in the supine position, with the head rotated. We do not use a headholder, although one may be used if greater posterior exposure is required. The operating room table is reversed so that the patient’s head is located on the foot of the bed; this allows the surgeon to sit and work comfortably during the procedure without any obstruction under the table. A long anesthesia circuit permits the anesthesiologist to stay at the other end of the surgical site. An electrically controlled table enables the frequent turning from side to side needed during neurotologic procedures.
The middle fossa approach facilitates the unroofing of the IAC and the exposure of the fundus of the canal. The facial nerve is located at the lateral end of the canal, where it enters the temporal bone and becomes the labyrinthine segment between the cochlea and the superior semicircular canal. This approach makes possible the removal of laterally located tumors in the IAC without the need for blind dissection.
The incision starts in the pretragal area, curves initially posteriorly above the ear, and then runs vertically for 4 cm before curving at a right angle anteriorly in the temporal area. The shape of the incision resembles a question mark (Fig. 16.1). Once the skin is elevated, an incision is made along the insertion of the temporalis muscle and fascia, and the muscle is reflected anterior inferiorly.
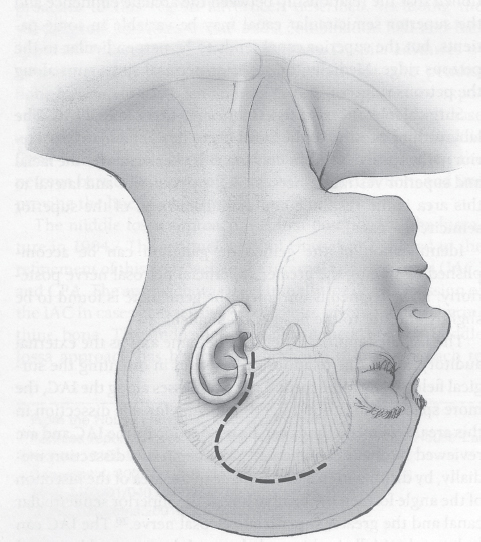
Fig. 16.1 The incision starts in the pretragal area, curves initially posteriorly above the ear, and then runs vertically for 4 cm before curving at a right angle anteriorly in the temporal area.
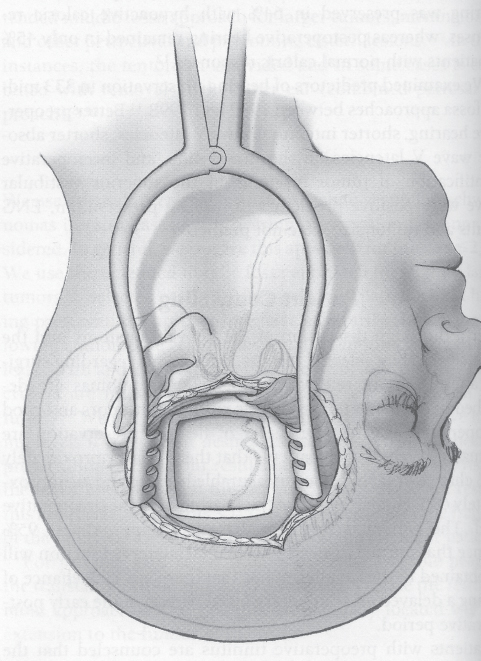
Fig. 16.2 The craniotomy measures 5 × 5 cm and is located two thirds anterior and one third posterior in relation to the external auditory canal.
Using a cutting bur, an opening is made in the squamous portion of the temporal bone. The craniotomy measures 5 × 5 cm and is located two thirds anterior and one third posterior in relation to the external auditory canal (Fig. 16.2). The bone flap is kept in antibiotic solution and is placed back at the conclusion of the surgery. The dura is now elevated from posteriorly to anteriorly from the floor of the middle fossa, and any remaining bone over the root of the zygoma is drilled away as close as possible to the floor of the middle fossa (Fig. 16.3). The initial landmark, the middle meningeal artery, marks the anterior extent of dural elevation. Frequently, bleeding is encountered in this area and is controlled by packing Surgicel into the foramen spinosum. Dissection of the dura continues until the petrous ridge is identified. Once the dura is completely elevated, the House-Urban retractor is placed into position over the porus acusticus (Fig. 16.4). At this point in time, the arcuate eminence and the greater superficial petrosal nerve have been identified. In a small proportion of patients, the geniculate ganglion is dehiscent, and care is taken not to injure it while elevating the dura. Posterior to anterior elevation avoids elevating the ganglion.
Using suction irrigation and diamond burs, dissection of the IAC is started medially. The IAC bisects the angle formed by the greater superficial petrosal nerve and the arcuate eminence as described by Bocca and García-Ibáñez.20 Identifying the IAC medially and anteriorly is safest because medially there are no important anatomic structures. Once the IAC is identified, bone surrounding it in the area of the porus is removed. Bone removal extends posteriorly to the level of the arcuate eminence and the common crus, anteriorly to Kawase’s triangle. Bone is removed 270 degrees around the canal, including the entire posterior lip. Lateral dissection of the IAC then proceeds. The exposure narrows laterally because of the presence of the cochlea anteriorly and the ampullated end of the superior semicircular canal posteriorly. At the lateral end of the canal, Bill’s bar is identified (Fig. 16.5). The facial nerve is followed into its labyrinthine portion. The ligament surrounding it at the beginning of the labyrinthine segment is cut to allow for decompression of the nerve in this portion.
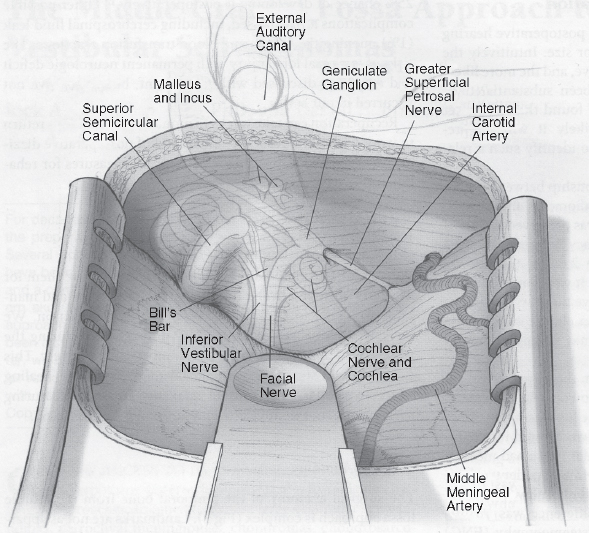
Fig. 16.3 Anatomy of the middle fossa floor.
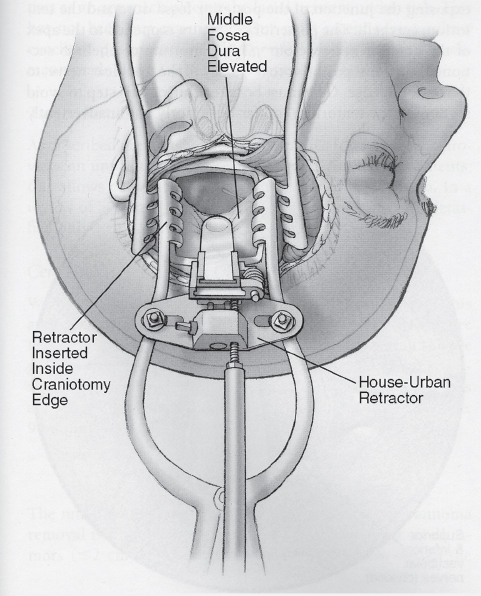
Fig. 16.4 Placement of the House-Urban retractor and exposure of the internal auditory canal.
The dura of the IAC is opened along the posterior aspect (Fig. 16.6). The facial nerve is identified clearly and stimulated. The superior vestibular nerve is cut at the end of the IAC. Following this, the vestibulofacial anastomotic fibers are cut. The tumor is then separated from the end of the IAC and from the facial nerve (Fig. 16.7). The goal is to free the tumor from the facial nerve and to deliver it from under the nerve. Dissection of the lateral end of the inferior compartment of the IAC can be very difficult. It is best to cut both superior and inferior vestibular nerves to avoid postoperative unsteadiness. Once the lateral end of the tumor has been delivered, the plane between the cochlear and facial nerves and tumor become apparent. This plane is developed using fine hooks. Tumor dissection proceeds from medial to lateral to avoid stretching the facial and cochlear nerves. At this point, a search for the anterior inferior cerebellar artery begins. Great care is taken to identify and not injure this important artery. At this point, the tumor is separated gently from this vessel. Debulking of the tumor begins, using small cup forceps. At all times, care is taken not to injure the facial nerve with the suction or by stretching it. Finally, the medial end of the tumor is freed with small hooks.
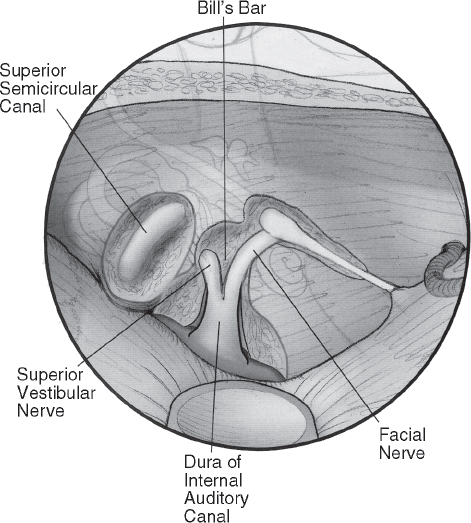
Fig. 16.5 The vertical crest or Bill’s bar separates the facial nerve from the superior vestibular nerve at the fundus of the internal auditory canal.
Papaverine pledgets are placed over the nerve and in the dissection field if changes in monitoring potentials are noted. This is thought to prevent vasoconstriction of the vessels feeding the inner ear and therefore enhancing chances at hearing preservation.
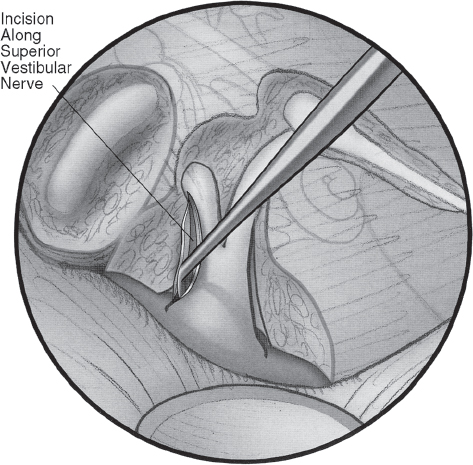
Fig. 16.6 The dura is incised along the posterior border of the dissection.
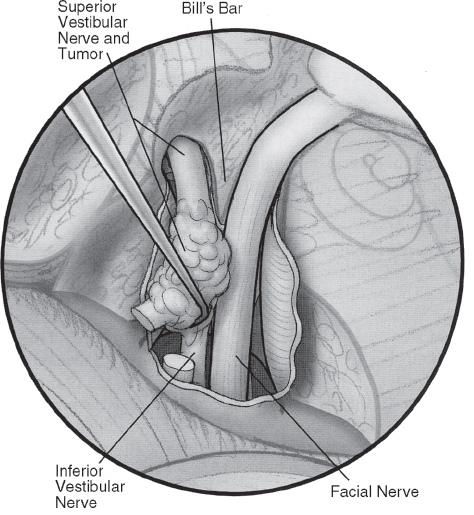
Fig. 16.7 The tumor is dissected away from both the facial and cochlear nerves.
Once tumor removal is completed, hemostasis is obtained (Fig. 16.8). The tumor bed is irrigated copiously. Abdominal fat is obtained and is used to close the dural defect. The temporal lobe retractor is removed. The dura is suspended on either side of the craniotomy to limit the dead space. A Penrose drain is placed into the wound. The bone flap is re-positioned and secured in place using three titanium micro-plates. The wound is closed in layers, and a mastoid-type pressure dressing is applied.
♦ Postoperative Care
The patient is observed in the intensive care unit for a period of 24 hours. Steroids and antibiotics are routinely used. For the middle fossa approach, the Penrose drain is removed from the wound on the first postoperative day. A new pressure dressing is applied. The wound is inspected every day thereafter. The mastoid dressing remains in place for 4 days and the patient is instructed not to lift or strain during the early postoperative period.
♦ Perioperative Complications
Slattery et al21 reviewed the perioperative morbidity of acoustic tumor surgery for a series of 1687 patients having surgery at the House Ear Institute (Los Angeles, CA) between 1987 and 1997. This included tumors of all sizes and using all surgical approaches. A CSF leak was the most common problem, occurring in 9.4% overall. Looking at the data from that study for just those tumors that underwent a middle fossa (n = 432) approach (mean size, 1.1 cm; min-max, 0.3–4.0), CSF leak occurred in 24 of the cases (5.6%), with reoperation required for treatment in only four cases (0.9% of all). In tumors operated with the middle fossa approach from 1998 through 2004 at the institute (n = 692), 4.4% had a CSF leak. Most leaks can be stopped with a pressure head dressing and bed rest with the patient’s head elevated. If the leak continues despite having the dressing in place, a lumbar spinal drain is inserted and kept in place for 3 to 4 days. Reexploration of the wound and repacking of the wound with additional fat is done if the leak persists despite the above steps. If the hearing is lost, the leak is controlled by performing a blind sac closure of the external auditory canal and obliteration of the middle ear space and eustachian tube using Surgicel and muscle.
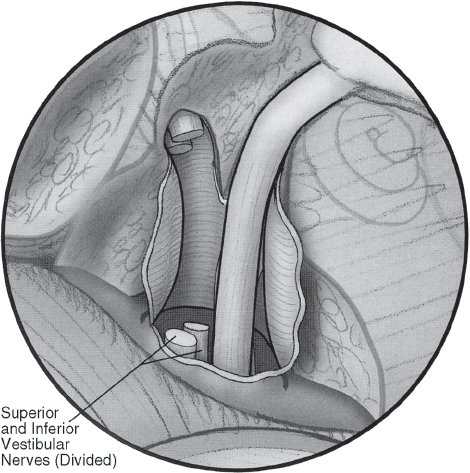
Fig. 16.8 Tumor removal is completed; the vestibular nerve is completely divided.
Meningitis is an uncommon complication and is managed with appropriate antibiotics following culture and identification of the offending organisms. Middle fossa cases from the Slattery et al study showed a prevalence of meningitis of 1.2%. For middle fossa cases of the past 7 years at the institute, meningitis occurred in 0.3%.
Although rare, the most common early postoperative complication is a hematoma in the CPA, occurring in 0.9% of all size tumors in the Slattery et al study and 0.7% of the middle fossa surgeries. This is manifested by signs of increased CPA pressure, and is managed by immediate opening of the wound and removal of the fat in the intensive care unit. The patient is then taken to surgery to secure hemostasis and repack the wound. The incidence of this complication is lowered by leaving a Penrose drain in the wound for the first 24 hours, and by obtaining meticulous hemostasis prior to closure of the wound.
Other perioperative complications occurring in the Slattery et al study for tumors removed by the middle fossa approach include craniotomy wound infection (1.7%) and cerebral edema (1.4%). There were no cases of hydrocephalus, embolus, thrombosis, abdominal wound infection, stroke, or death.
If facial weakness occurs, the eye is protected by using conservative measures first. These include artificial tears, moisture chambers, and soft contact lens. In certain situations, the insertion of a gold weight or a palpebral spring may be necessary. In middle fossa cases from the Slattery et al study, 1.4% required one of these surgical procedures, as did 0.9% of the cases in the 1998–2004 series.
Possible problems related to temporal lobe retraction include memory loss, auditory hallucinations, and speech disturbances. These are rarely significant clinical problems. Seizures are often cited as a possible complication of the middle fossa approach, but in reality this problem is rarely encountered. Older patients do not tolerate the middle fossa approach as well as younger patients due to the fragility of the dura and retraction of the temporal lobe. Fortunately, all of the above complications have been very rare in our experience.
♦ Results
Complete tumor removal has been demonstrated in more than 95% of patients undergoing microsurgical resection, and was accomplished in 94.6% of all middle fossa surgeries at the House Ear Institute (n = 1183) and 95.1% of the 1998– 2004 series. Tumor recurrence has been shown to occur in 0.3% or less of cases with long-term follow-up.22 Cranial nerve morbidity, particularly of cranial nerves VII and VIII, is of particular concern when judging the success of any form of treatment modality for vestibular schwannomas.
Facial Nerve
Previous publications from the House Ear Institute have reported a follow-up (1-year) rate of 95.8% good facial function (House-Brackmann grades I or II) in tumors less than or equal to 1.5 cm in size and removed using the middle fossa approach, a rate that was not statistically significantly different from that of the translabyrinthine cases.7 For tumors 1 cm or less in size, results were similar. For the recent 7-year period, 692 tumors underwent middle fossa removal, with a good facial function rate of 90.2%, including all tumor sizes (up to 6 cm, with 16% >1.5 cm).
Others have reported facial nerve results for small tumors removed using the middle fossa approach. For example, Satar et al23 reported rates of good facial function (House-Brackmann grades I or II) in 93.7% of 64 intracanalicular tumors and 97.6% of 42 tumors with 1 to 9 mm CPA extension.
Hearing Preservation
For middle fossa procedures, hearing preservation is a goal. Slattery and Brackmann24 reviewed the published results of hearing preservation for both the suboccipital/retrosigmoid and the middle fossa approaches. As they note, there are different definitions of hearing preservation, including simply preserving the cochlear nerve, any measurable hearing, serviceable hearing, or lack of change in hearing from the preoperative level. Hearing preservation rates, as reported by the various authors reviewed, ranged from 16.5% to 65% for the suboccipital approach and 36% to 71% for the middle fossa approach. Tumors included in these studies ranged from intracanalicular only in some studies to as large as 3 cm in one study.
A report from the House Ear Institute on all tumors operated using the middle fossa approach over a 2½-year period from 1993 to 1995 found measurable hearing preserved in 68% of 143 patients.6 Hearing was preserved within 15 dB pure tone average (PTA) and 15% speech discrimination score (SDS) of preoperative levels in 52%; 47% did not change or improved their hearing class using the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) classification system. In a more recent paper from the institute, hearing preservation was evaluated by tumor size for patients operated using the middle fossa approach between 1992 and 1998.19 Again, no effect of tumor size on hearing outcome was found. But for 107 tumors of 1 cm or less in size, some measurable hearing was preserved in 79%, and serviceable hearing (equal to or better than 50 dB PTA and 50% SDS) was preserved in 60%. Hearing was preserved to within 15 dB and 15% of preoperative levels in 52%. For the most recent 7-year series, 59.1% of the 428 patients with audiologic data available to compute the AAO-HNS class had class A or B results (50 dB/50%), whereas 73.2% had measurable hearing. Long-term stability of hearing preservation in our middle fossa vestibular schwannoma cases has also been examined. Friedman et al25 found that 70% of patients with immediate postoperative serviceable hearing maintained serviceable hearing at more than 5 years after surgery.
Others have also reported hearing preservation rates in small tumors. Staecker et al26 reported a hearing preservation rate of 57% in mainly intracanalicular tumors removed by the middle fossa approach and 47% in similar tumors removed using the retrosigmoid approach. Satar et al23 reported rates of functional hearing preservation in intracanalicular tumors and those with up to 9 mm CPA extension of 62% and 63%, respectively. These studies, as well as our own data, all show preservation of serviceable hearing in approximately 60% of patients undergoing middle fossa acoustic tumor removal for small tumors.
♦ Conclusion
The middle cranial fossa approach is a technically demanding approach. It remains our approach of choice for patients with small acoustic tumors and serviceable hearing. Hearing preservation rates are approximately 60 to 70%. Results on the facial nerve outcomes are no different from those for other more traditional approaches. The safety and efficacy of the middle fossa approach in the area of acoustic tumor removal, our main indication, has been clearly demonstrated over the years.
References
1. House WF. Surgical exposure of the internal auditory canal and its contents through the middle, cranial fossa. Laryngoscope 1961;71:1363–1385 PubMed
2. House WF. Middle cranial fossa approach to the petrous pyramid. Report of 50 cases. Arch Otolaryngol 1963;78:460–469 PubMed
3. House F, Hitselberger WE. The middle fossa approach for removal of small acoustic tumors. Acta Otolaryngol 1969;67:413–427 PubMed
4. Shelton C, Brackmann DE, House WF, Hitselberger WE. Acoustic tumor surgery. Prognostic factors in hearing conversation. Arch Otolaryngol Head Neck Surg 1989;115:1213–1216 PubMed
5. Gantz BJ, Parnes LS, Harker LA, McCabe BF. Middle cranial fossa acoustic neuroma excision: results and complications. Ann Otol Rhinol Laryngol 1986;95(5 Pt 1):454–459 PubMed
6. Slattery WH III, Brackmann DE, Hitselberger W. Middle fossa approach for hearing preservation with acoustic neuromas. Am J Otol 1997;18: 596–601 PubMed
7. Arriaga MA, Luxford WM, Berliner KI. Facial nerve function following middle fossa and translabyrinthine acoustic tumor surgery: a comparison. Am J Otol 1994;15:620–624 PubMed
8. Brackmann DE. Translabyrinthine removal of acoustic neurinomas. In: Brackmann DE, ed. Neurological Surgery of the Ear and Skull Base. New York: Raven Press, 1982:235–241
9. House WF, Luetje CM, eds. Acoustic Tumors, Vol 1: Diagnosis. Baltimore: University Park Press, 1979
10. Roberson JB Jr, Brackmann DE, Hitselberger WE. Acoustic neuroma recurrence after suboccipital resection: management with translabyrinthine resection. Am J Otol 1996;17:307–311 PubMed
11. Briggs RJ, Luxford WM, Atkins JS Jr, Hitselberger WE. Translabyrinthine removal of large acoustic neuromas. Neurosurgery 1994;34:785–790, discussion 790–791 PubMed
12. Krause F. Zur Freilegung der hinteren Felsenbeinflache und des Kleinhirns. Beitr Klin Chir 1903;37:728–764
13. Shelton C, Alavi S, Li JC, Hitselberger WE. Modified retrosigmoid approach: use for selected acoustic tumor removal. Am J Otol 1995;16: 664–668 PubMed
14. Smith PG, Leonetti JP, Grubb RL. Management of cerebrospinal fluid otorhinorrhea complicating the retrosigmoid approach to the cerebellopontine angle. Am J Otol 1990;11(3):178–180 PubMed
15. Schessel DA, Nedzelski JM, Rowed DW, Feghali JG. Headache and local discomfort following surgery of the cerebellopontine angle. In: Tos M, Thomsen J, eds. Acoustic Neuroma. Proceedings of the First International Conference on Acoustic Neuroma. Amsterdam: Kugler, 1991: 899–904
16. House JL, Hitselberger WE, House WF. Wound closure and cerebrospinal fluid leak after translabyrinthine surgery. Am J Otol 1982;4:126–128 PubMed
17. Tos M, Thomsen J. Cerebrospinal fluid leak after translabyrinthine surgery for acoustic neuroma. Laryngoscope 1985;95:351–354 PubMed
18. Shelton C, Brackmann DE, House WF, Hitselberger WE. Middle fossa acoustic tumor surgery: results in 106 cases. Laryngoscope 1989;99: 405–408 PubMed
19. Brackmann DE, Owens RM, Friedman RA, et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol 2000;21:417–424 PubMed
20. Bocca E, García-Ibáñez E. Surgical anatomy of the internal auditory meatus. Acta Otorinolaryngol Iber Am 1969;20:233–245 PubMed
21. Slattery WH III, Francis S, House KC. Perioperative morbidity of acoustic neuroma surgery. Otol Neurotol 2001;22:895–902 PubMed
22. Shelton C. Unilateral acoustic tumors: how often do they recur after translabyrinthine removal? Laryngoscope 1995;105(9 Pt 1):958–966 PubMed
23. Satar B, Jackler RK, Oghalai J, Pitts LH, Yates PD. Risk-benefit analysis of using the middle fossa approach for acoustic neuromas with >10 mm cerebellopontine angle component. Laryngoscope 2002;112(8 Pt 1): 1500–1506 PubMed
24. Slattery WH, Brackmann DE. Hearing preservation and restoration in CPA tumor surgery. Neurosurg Q 1997;7:169–182
25. Friedman RA, Kesser B, Brackmann DE, Fisher LM, Slattery WH, Hitselberger WE. Long-term hearing preservation after middle fossa removal of vestibular schwannoma. Otolaryngol Head Neck Surg 2003;129:660–665 PubMed
26. Staecker H, Nadol JB Jr, Ojeman R, Ronner S, McKenna MJ. Hearing preservation in acoustic neuroma surgery: middle fossa versus retrosigmoid approach. Am J Otol 2000;21:399–404 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








