The art in smears lies in three domains: the physical preparation of the smear, perceiving and recognizing structure at different powers of examination, and knowing the origin and limitations of the information provided. This chapter discusses the limitations and some problems associated with smears.
In the “frozen section” room, a pathologist must decide whether to smear, freeze, or do both. Smearing tissue entails careful observation followed by sampling of the specimen. The glass slide will contain a subpopulation of hopefully representative cells but not the entire biopsy. For this reason, it is usually inadequate for evaluating a “margin.” For proper assessment, a surgical margin needs to be examined in its entirety, not selectively sampled. If it is tiny, the whole specimen can be smeared. Otherwise it will require freezing and cutting the entire biopsy. Some types of tissue defy smearing, especially those having extensive reticulin or collagen such as many schwannomas, hemangioblastomas, and the rare sarcoma. Crushing and smearing destroys low-magnification architecture. It is just this information that is diagnostic for cystic lesions such as colloid cysts; they require freezing, because their recognition requires macroscopic structure. Also, the grumose material within a dermoid or epidermoid cyst will stubbornly refuse to remain on the glass slide, in either a smear or frozen section. Finally, tumors in which the low-power architecture is key to the diagnosis, such as craniopharyngioma, often require a frozen section for a confident evaluation.
Artifacts and Problems
Artifacts abound in pathology. These range from how a surgeon or pathologist samples a lesion, through the processing of tissue, to dust on a microscope’s ocular. Artifacts are important to recognize so as to avoid confusion. An experienced pathologist often no longer “sees” the artifacts that frequently confuse the novice. Knowing how artifacts arise often provides useful information. For example, halos in oligodendrogliomas are processing artifacts but give structural information about the tumors; in this case, the halos reflect the relative paucity of cytoskeletal elements.
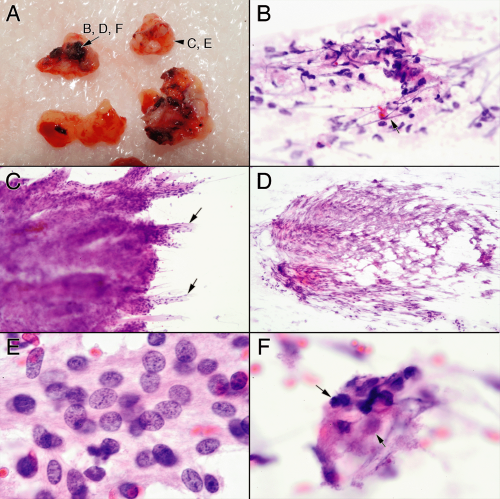
During an operation, aside from the obvious and inevitable sampling artifacts, how the surgeon handles the tissue can induce selective difficulties, including cautery and crush effects. Cautery greatly augments surgery and burdens the pathologist. Being asked to make a diagnosis on cauterized tissue is akin to eating an incinerated burger at a friend’s cookout; you swallow it and try to be cordial. Burnt embers of tissue are easy to recognize and enable the pathologist to add appropriate, if slightly nasty, qualifiers to a report (e.g., “Cautery artifacts preclude optimal evaluation.”) However, electrocution takes many forms, from grilled embers to subtle structural and cytological changes that can truly confound a diagnosis. For this reason, knowing the spectrum of changes wreaked by cauterization is important. Cautery effects typically do not follow anatomically meaningful boundaries. Electrical currents travel long distances along vessels, much further than in the surrounding parenchyma; look to see if small vessels are odd in color, folded, or are “smudged.” In a smear, look for unexplained loss of nuclear detail (e.g., not due to air drying or necrosis; Figure 13-1). Nuclei subjected to high voltage more easily lose their integrity and easily streak across the slide. The combination of smudged but not necrotic nuclei and streaked nuclei suggests cautery effects. Decimated nuclei intermixed with stroma impart a myxoid appearance to the smear that resembles necrosis. Even in permanent sections, malignant areas can be converted to a benign morphology
and Antoni A areas can be converted to Antoni B areas (Figure 13-2). Cautery transforms meningioma whorls into amorphous excrescences, beautiful nuclear cytology into pyknotic, crenated smudges (Figure 13-3). Be cautious about making a major diagnosis on cauterized tissue; it can deceive. The best course of action is to ask the surgeon to send out noncauterized tissue for a more accurate diagnosis. Just because the tissue is not crisp does not mean the cautery was silent.
and Antoni A areas can be converted to Antoni B areas (Figure 13-2). Cautery transforms meningioma whorls into amorphous excrescences, beautiful nuclear cytology into pyknotic, crenated smudges (Figure 13-3). Be cautious about making a major diagnosis on cauterized tissue; it can deceive. The best course of action is to ask the surgeon to send out noncauterized tissue for a more accurate diagnosis. Just because the tissue is not crisp does not mean the cautery was silent.
Bone dust can occasionally contaminate a specimen. Minute particles of bone embedded in biopsy material mimic calcifications in a smear. Mineralization is common in low-grade tumors, so such deposits can suggest an erroneous benign or low-grade diagnosis. Also, do not confuse dust with psammoma bodies; the latter are aesthetic laminated concretions, the former irregular, jarring, dark fragments.
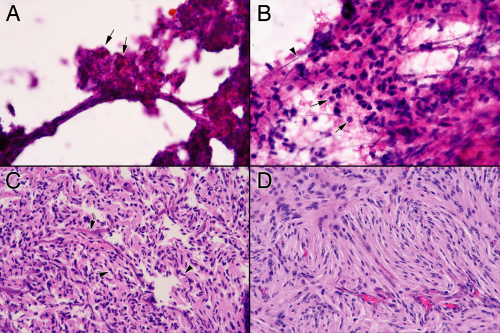
 FIGURE 13-2. Cautery artifacts in schwannoma permanent sections. These panels are from the same tumor as in Figure 13-1. A. Parts of this schwannoma have diagnostic Verocay bodies, with their alternating palisades of tumor and collagenous zones (arrows). B. The low-magnification view at first glance appears like Antoni B areas ringed by Antoni A zones. However, the rings are too perfect. Region a has extensive cautery artifacts at high power, region b has less, whereas electrical current to region c has lead to extensive cellular vacuolization. Only region d is near normal. C. Comparison of nuclei in a cauterized region (a) with that in a slightly better preserved region (b). In the cauterized region, nuclei are enlarged and smudgy. Compare region a to Figure 13-1D. D. Electrical current flows better along tissue than perpendicular to it. Consequently, it can flow for longer distances down vessels, leaving smudgy structures (arrow) even when nearby nuclei seem less disturbed (arrowhead). Without the affected vessel, the nearby dark, featureless nuclei (arrowhead) could be ascribed incorrectly to processing, rather than cautery. |
Preparing a smear creates its own set of artifacts. As mentioned previously, scrutinize the biopsy and sample everything that looks different. If necessary, make two smears. A dark burgundy blood clot could really be a solid melanoma. The tan-yellow material that looks a bit like necrosis could really be just cortex. A tissue’s appearance does not deceive, only its interpretation. Using your eyes
is a key strength of a smear; ignoring what you see diminishes its accuracy. Never trust forceps; always clean them carefully before use. Bits of colon cancer from opening the previous bowel resection will jump out of a smear and divert the eyes away from the real pathology.
is a key strength of a smear; ignoring what you see diminishes its accuracy. Never trust forceps; always clean them carefully before use. Bits of colon cancer from opening the previous bowel resection will jump out of a smear and divert the eyes away from the real pathology.
Streaked out or extruded nuclei are occasionally useful but are usually just annoying and distracting (Figure 13-4). A nucleus will streak when external shearing forces exceed the strength of its complex internal architecture. Given a great enough external force, any nucleus will break down; given a weak enough internal structure, even minimal force will streak a nucleus. Like a house of cards, a nucleus usually either stands intact or completely collapses; partially streaked nuclei are uncommon. Cells with weak nuclei include those from tumors having scant cytoplasm (e.g., small cell carcinomas, medulloblastomas), recently necrotic cells that have not yet undergone coagulation of their proteins, and those that have been electrocuted. Excessive external forces arise in biopsy clamps that the surgeon uses to yank samples from rubbery masses as well as in the crush-and-smear phases of a smear preparation. Because of these many variables, streaked nuclei alone are usually not useful. However, nuclei in normal or reactive tissue typically have great internal strength and resist streaking, even as nearby invading tumor cells smear across the field. Many streaked nuclei in a small-round blue cell tumor favor a neuroendocrine or primitive neuroectodermal tumor over hardier lymphomas.
Three difficulties frequently confound a novice staining a brain smear: insufficient eosin staining, air drying,
and bubbles under the coverslip. Especially in brain biopsies, a smear lacking sufficient eosin fails to show the fine glial matrix underlies many different pathologies (Figure 13-5). Differentiating reactive gliosis from a neoplastic glioma or metastatic tumor from a glioblastoma requires examining how the eosinophilic matrix relates to the offending cells. In a smear, eosin strongly stains astroglial fibers. When absent, the fibers become pale blue or disappear into the background artifacts. A brain smear never had too much eosin.
and bubbles under the coverslip. Especially in brain biopsies, a smear lacking sufficient eosin fails to show the fine glial matrix underlies many different pathologies (Figure 13-5). Differentiating reactive gliosis from a neoplastic glioma or metastatic tumor from a glioblastoma requires examining how the eosinophilic matrix relates to the offending cells. In a smear, eosin strongly stains astroglial fibers. When absent, the fibers become pale blue or disappear into the background artifacts. A brain smear never had too much eosin.
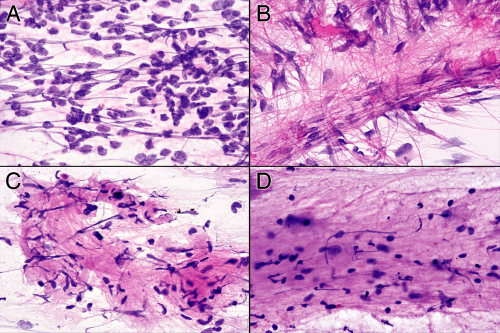 FIGURE 13-4. Streaked nuclei. These four panels show nuclei that have streaked out during the smear. A. Medulloblastoma. Many of the nuclei from the small, undifferentiated cells have squished out across the field. B. Ependymoma. Notice how some of the streaked nuclei follow the dense glial fibers arising from the vessel. C
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|