Fig. 18.1
Mixed adenoneuroendocrine carcinoma (MANEC) of the pancreas. (a) In the left half of the image, the pancreatic ductal adenocarcinoma component is composed of well-differentiated glandular structures within desmoplastic stroma (hematoxylin and eosin stain, magnification ×40). In the right half of the image, neuroendocrine carcinoma tumor cells have a high nuclear-to-cytoplasmic ratio with focal comedo necrosis. (b) The neuroendocrine cells are positive for chromogranin while the adenocarcinoma component is negative (magnification ×40)
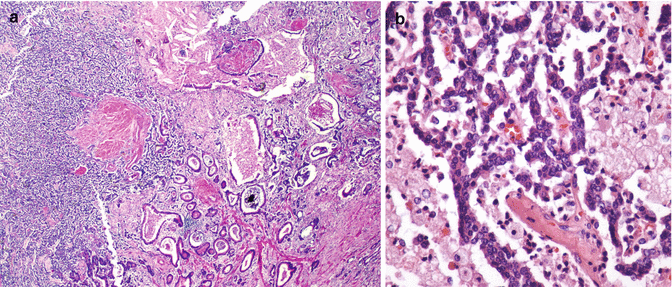
Fig. 18.2
(a) Mixed adenoneuroendocrine tumor (MANET) of the pancreas. In the right half of the image, the adenocarcinoma component is composed of well-differentiated glands within desmoplastic stroma (hematoxylin and eosin stain, magnification ×20). In the left half of the image, a second population of neuroendocrine tumor cells is seen which has high nuclear-to-cytoplasmic ratio and anastomosing ribbon-like network. (b) Image shows that these neuroendocrine cells are bland with only mild atypia (hematoxylin and eosin stain, magnification ×200)
18.3 Clinicopathologic Features
Patients with pancreatic MANEC range in age from 21 (unpublished data) to 84 years (mean of 68 years) and males and females are equally affected [5, 12–14, 18–25]. Tumors may arise anywhere in the pancreas, but are most common in the head, followed by the tail and body. They are often fairly cellular circumscribed tumors and range in size from 2 to 12 cm (mean 5.6 cm) [5, 13, 19].
18.3.1 Diagnosis and Morphology
The distribution of the two constituent tumor types is highly variable in MANECs. The two components may occur as separate and distinct (Fig. 18.1) or composite foci [4, 13, 19] or may be more intricately mixed (Fig. 18.3). The former group (the presence of distinct components) has also been termed “collision” tumors in the literature, but we prefer to reserve this term for neoplasms in which the two components are believed to be independent of each other (such as a serous adenoma and a NE tumor), presumably arising from different cell types. In contrast, in MANECs, the two components seem to be the product of the same neoplasm, even though they may appear as, and form distinct foci.


Fig. 18.3
Mixed adenoneuroendocrine carcinoma (MANEC) of the pancreas. Malignant glands are intimately admixed with large neuroendocrine cells showing high nuclear-to-cytoplasmic ratio and coarse salt-and-pepper chromatin (hematoxylin and eosin stain, magnification ×200)
In some MANECs, the two cell types may not be clearly recognizable as distinct on cursory histologic examination and may require immunostains to more clearly identify a dual or divergent immunoprofile, while in other cases, the dual differentiation may be identified within the very same cells (true “amphicrine” carcinomas).
The ductal adenocarcinoma (PDAC) component of pancreatic MANECs typically shows variable differentiation ranging from well-differentiated tumors with well-formed glands (Fig. 18.1) to poorly differentiated carcinomas with ill-defined glandular units (Fig. 18.3), as well as clusters and sheets of highly malignant epithelial cells with marked nuclear pleomorphism and prominent nucleoli. These are typically embedded in abundant desmoplastic stroma. High-grade pancreatic intraepithelial neoplasia (PanIN-3) may be identified in the background pancreatic parenchyma [5].
The NE component is more frequently a poorly differentiated neuroendocrine carcinoma (PDNEC) of small or large cell type, as defined in the lungs. The small cell type is characterized by sheets or islands of small cells with high nuclear-to-cytoplasmic ratio, hyperchromatic nuclei with coarse chromatin, inconspicuous nucleoli, and irregular nuclear contours, with nuclear molding, single-cell and confluent necrosis, and brisk mitotic activity (>20/10 high power fields). The NE nature of the large cell type is often more readily evident in many examples by the more nested pattern and cytologic uniformity characteristic of NE carcinomas and is also often more successfully highlighted by immunohistochemical evaluation. Typically large cell types have more abundant cytoplasm and large nuclei with open chromatin and prominent nucleoli.
Among the six pancreatic MANECs described by Basturk et al. [5], five had a large cell PDNEC component and one had a small cell PDNEC component. Four of the tumors showed lymphovascular and perineural invasion, as well as positive resection margins. All tumors had lymph node metastasis and by Union for International Cancer Control (UICC) guidelines were stage pT3N1 with two showing liver metastasis at diagnosis.
18.3.2 Immunohistochemistry
The PDAC component expresses a range of keratins including pancytokeratin, cytokeratin 7, and Cam5.2 as well as the glycoproteins of carcinoembryonic antigen (CEA), CA19-9, MUC1, and B72.3 [23]. Poorly differentiated forms show nuclear positivity for p53 [13, 26] (indicative of p53 gene mutation), loss of DPC4 (indicative of DPC4 gene mutations), and brisk mitotic activity corresponding to high Ki-67 indices [5]. The epithelial cells of glandular origin typically do not express NE markers.
In contrast, the NE cells in the NE component of the tumor, whether well or poorly differentiated, often express NE markers (synaptophysin, chromogranin A, and CD56), typically in more than 10 % of the tumor cells (Fig. 18.1), in addition to epithelial markers such as pancytokeratin. NE marker expression may be sparser in higher-grade examples. Synaptophysin is more sensitive than chromogranin A, but the latter is more specific [4]. CD56 antibody targets neural cell adhesion molecules (NCAMs), and although it is the least specific neuroendocrine marker, it may be useful in the differential diagnosis of these tumors. TTF1 is also often expressed in the PDNEC component, especially in those with a small cell pattern. p53 is positive in the NE cells and Rb is lost in up to 90 % [27]. In fact, some advocate the use of Rb loss as evidence of NE lineage in this setting. The pancreatic duodenal homeobox protein 1 (PDX-1) immunostain may also be positive in up to 40 % of PDNECs. BCL2 protein is also positive in PDNECs (100 % in small cell types and 50 % in large cell types), but is negative or focally positive in WDNETs [27]. By definition, the Ki-67 labeling index is typically high (greater than 50 %) in PDNECs and in fact is typically even greater than 80 % [5] but is between 0 and 20 % in WDNETs [17].
18.3.3 Cytopathology
The cytologic features of MANEC have only rarely been described [11, 22]. Tumors have both glandular and neuroendocrine features, the amount of which is highly variable depending on the component sampled during fine needle aspiration. This has significant implications for the accurate cytologic diagnosis of these tumors and may partly explain their underrepresentation in the cytology literature.
The ductal component has a variety of features depending on the degree of differentiation. For well-differentiated PDAC, tumor cells are usually present in flat or folded sheets composed of focally crowded and overlapping bland-appearing cells with only slight nuclear membrane irregularity and hyper- or hypochromasia. For poorly differentiated PDAC, cells form three-dimensional groups with nuclear pleomorphism, anisonucleosis, high nuclear-to-cytoplasmic ratio, marked nuclear contour irregularity, hypo- or hyperchromasia, and prominent (sometimes macro-) nucleoli. Single intact malignant cells, single-cell necrosis, abnormal mitotic figures, and background necrosis are typically seen in poorly differentiated tumors [11].
The neuroendocrine component also shows variable cytology depending on the degree of differentiation of the tumor. For tumors with a WDNET component, tumor cells are typically singly dispersed and bland, with plasmacytoid features. Focal rosettes may be seen both on cell blocks and on smears [11]. Nuclei are round to oval and bland and on Papanicolaou stain have characteristic salt-and-pepper chromatin. Nucleoli, if present, are usually small in well-differentiated neuroendocrine tumors. In small cell-type PDNEC, tumor cells resemble their counterparts in the lung. They are small to intermediate with high nuclear-to-cytoplasmic ratio, inconspicuous nucleoli, nuclear contour irregularity, salt-and-pepper chromatin, nuclear molding, single-cell and confluent necrosis, as well as crush artifact. Large cell-type PDNEC is characterized by large cells with abundant cytoplasm and nuclei with open, vesicular chromatin, and prominent nucleoli. The latter may be especially difficult to distinguish from the poorly differentiated PDAC component of the tumor without the help of immunohistochemical stains.
18.4 Differential Diagnosis
18.4.1 Ordinary Pancreatic Ductal Adenocarcinoma with Isolated Neuroendocrine Cells
PDACs can have rare isolated nonneoplastic neuroendocrine cells (best identified by immunohistochemical staining). In most instances, they are represented as rare scattered cells and, by definition, account for far less than 30 % of the tumor volume [4, 23]. These should not be categorized as MANECs nor should NE neoplasms that have focal non-neuroendocrine ductal cells.
It should be noted here that in many PDACs, adenocarcinoma cells invade into, and partially replace, the native islets of Langerhans and are intimately admixed with the islet cells (peri-isletic invasion; Fig. 18.4). When the adenocarcinoma is well differentiated and subtle, these foci can be dismissed as benign ductulo-insular complexes. Conversely, when carcinoma is prominent and massively replaces the islet, the remaining islet cells can be mistaken as evidence of “mixed” differentiation.
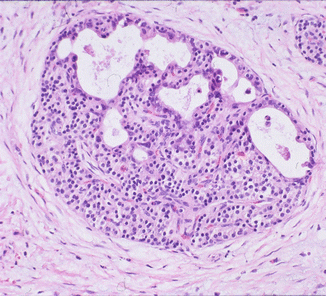

Fig. 18.4
Pancreatic ductal adenocarcinoma with malignant glands invading and partially replacing a native islet of Langerhans, the so-called peri-isletic invasion (hematoxylin and eosin stain, magnification ×100)
18.4.2 Pancreatic “Ductulo-Insular”-Type Neuroendocrine Tumors
Some otherwise classical pancreatic neuroendocrine neoplasms show a striking admixture of benign ductular units. This phenomenon is known by a variety of names including pancreatic neuroendocrine tumors with ductulo-insular differentiation, duct-islet cell tumor, mucin-producing islet cell adenoma, and ductulo-insular tumors. These NE tumors are typically well differentiated with classical morphology, and the ducts are generally believed to be benign native ducts (Fig. 18.5) that are proliferative (hyperplastic), presumably under the induction of local tumor factors [23, 28–30]. Although some authors had speculated in the past on the neoplastic nature of the ducts [29], more recent studies showed that the ductal cells were morphologically, immunohistochemically (p53-negative), and genetically nonneoplastic (negative for KRAS, DPC4, and ERBB2 mutations, all early genetic events in the development of PDAC) and likely represent preexisting entrapped ductules [30]. This is in contrast to MANECs in which both the glandular and neuroendocrine components are not only neoplastic but also malignant. Additionally, when ductulo-insular tumors metastasize, it is only the neuroendocrine component that metastasizes, unlike MANECs in which either or both components may metastasize [14, 22]. Therefore, the interchangeable use of the terminology mixed ductular-insular tumor and MANECs is incorrect and misleading since the latter are morphologically malignant tumors with uniformly poor prognosis. In MANECs, the coexistence of ductular and neuroendocrine cells has been postulated to be due to transdifferentiation of neuroendocrine cells or divergent differentiation of pancreatic stem cells along ductal and endocrine lines.
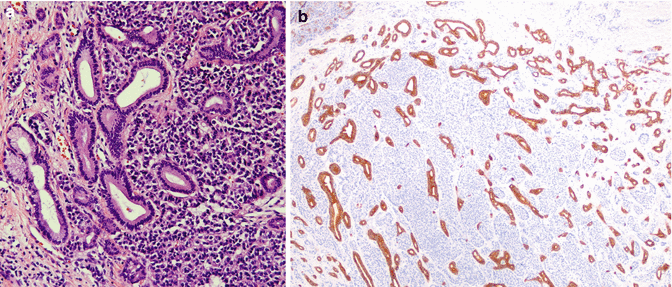

Fig. 18.5
Ductulo-insular tumor of the pancreas. (a) Neuroendocrine tumor cells are dispersed as aggregates of plasmacytoid cells with high nuclear-to-cytoplasmic ratio. Benign pancreatic ductules surround them peripherally (hematoxylin and eosin stain, magnification ×200). (b) The benign ductules are positive for cytokeratin 19, while the well-differentiated neuroendocrine component is negative (magnification ×40)
This phenomenon of ductulo-insular NETs (in which there are benign ductules within a WDNET) is reported in variable amounts in 16 % of all neuroendocrine tumors in the pancreas; however, the examples with more abundant ductal units as illustrated in Fig. 18.5 are far less common in our experience. They are typically smaller than classical pancreatic neuroendocrine tumors (<2 cm), have prominent intratumoral sclerosis, and are insulin-positive (by immunohistochemistry), and the benign ductules can be distributed centrally, peripherally, or diffusely within the tumor. Ductules stain positively with cytokeratin 7 and 19 and are negative for neuroendocrine markers [28, 29].
18.4.3 Pancreatoblastoma
Pancreatoblastoma which is an extremely rare primary pancreatic neoplasm that shows trilineage differentiation can show predominant ductal and NE differentiation, thus simulating a MANEC on histology. Although pancreatoblastomas are generally regarded as tumors of childhood, they certainly also occur in adults, with a second peak in the mid-30s. In addition to glandular and neuroendocrine components, tumors typically have a significant acinar component, which would stain for pancreatic enzymes trypsin and chymotrypsin, as well as BCL10. More importantly squamoid morules, which are meningothelial-like or squamoid cell clusters, often but not always with optically clear biotin rich nuclei [31], are pathognomonic of pancreatoblastoma.
18.5 Molecular Pathology
Unfortunately few definitive molecular studies have been done on MANECs. The genetic alterations in these tumors thus would have to be extrapolated from the work that has previously been done on ordinary PDACs and neuroendocrine neoplasms.
A variety of somatic mutations involving four key driver genes have been implicated in PDAC including KRAS, P16/CDKN2A, TP53, and SMAD4/DPC4 [32]. The frequency of these genetic mutations is somewhat variable, with KRAS and p16/CDKN2A mutations being most frequent (over 90 % of PDACs), while TP53 (75 %) and SMAD4/DPC4 (55 %) mutations are more variable.
Small and large cell-type PDNECs often show abnormal immunolabeling with p53 and Rb proteins (95 % and 74 % of cases, respectively) [27]. This correlates with intragenic mutant TP53 and retinoblastoma RB–1 genes. As a result p53 immunostain is frequently positive in the PDNEC component. Rb protein is lost in 60–90 % of PDNECs and tumors that retain Rb usually show concomitant loss of p16 staining [27], unlike WDNETs, in which Rb and p16 are retained. PDNECs retain DAXX and ATRX protein on immunohistochemical staining, indicative of the absence of inactivating DAXX (death-domain associated protein) and ATRX (alpha thalassemia/mental retardation syndrome X-linked) gene mutations. This is in contrast to WDNETs in which these mutations are frequently present and result in loss of DAXX and ATRX staining [33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







