Fig. 14.1
The basic lesion of most children with myelomeningocele is the open neural placode. The dorsal surface is the interior of the neural tube while the ventral surface is what would have been the entire outside of the neural tube had it closed. There is no skin overlying the defect and the placode is covered by an extremely thin arachnoid that will breakdown shortly after birth allowing infection, meningitis, and death if closure is not performed. Passing down the center of the placode is a narrow groove that is continuous with the central canal of the closed spinal cord. Cerebral spinal fluid passing down the central canal is discharged through a small opening at the upper end of the placode and bathes the external surface of the neural tissue (Reprinted from Lindseth [45]. With permission from Lippincott Williams & Wilkins)
In most neuromuscular diseases, the neurological status is predictably either stable or progressive. The clinical course of myelomeningocele is far less certain since a number of different central and peripheral nervous system abnormalities may be present. In addition to the spinal cord lesion with an established neurological level present at birth, there will usually be the Arnold–Chiari malformation and hydrocephalus and quite often syringomyelia and symptomatic spinal cord tethering. These additional problems will vary in their severity and may or may not cause neurological deterioration and orthopaedic deformities. They often require surgical treatment. When not causing neurological complications, they have the potential to do so during spinal surgery. Associated systemic anomalies occur (Table 14.1) and in some cases the defect is part of a syndrome involving chromosomal or single gene abnormalities (Table 14.2).
Table 14.1
Systemic anomalies associated with spina bifida
Skeletal | Gastrointestinal | Pulmonary | Craniofacial | Cardiovascular | Genitourinary |
|---|---|---|---|---|---|
Clubfeet, vertical talus + other foot deformities Lower extremity contractures Hip dislocation Scoliosis Kyphosis Spondylolisthesis Pectus excavatum Syndactaly Rib anomalies Charcot arthropathy | Inguinal hernia Mickel’s diverticulum Malrotation Omphalocele Imperforate anus | Tracheoesophageal Fistula Situs inversus | Synostosis Cleft palate Stabismus Low-set ears Hypertelorism | Ventriculo-septal defect Atrial-septal defect Patent ductus Coarcation | Hydronephrosis Hydroureter Horseshoe kidney Undescended testes Hydrocle Malrotation Exstrophy |
Table 14.2
Recognized syndromes including a neural tube defect
Genetic syndromes |
Meckel |
Median cleft face |
Robert’s |
Anterior sacral meningomyelocele and anal stenosis |
Trisomy-13 |
Trisomy-18 |
Triploidy |
Others including unbalanced translocation and ring chromosome |
Nongenetic syndromes |
Syndrome of the amnios rupture sequence |
Oculoauriculovertebral dysplasia |
Myelomeningocele was a uniformly fatal diagnosis until the second half of the twentieth century and the advent antibiotic therapy and advances in urological and neurosurgical care. A seminal event, and an example of the remarkable commitment seen in the families of children with myelomeningocele, is the development of a shunt for the treatment of hydrocephalus. In 1955, John Holter was working as a technician in a hydraulics factory when his first son, Casey, was born with myelomeningocele and hydrocephalus. With encouragement of Dr. Eugene Spitz, a neurosurgeon, Holter dedicated himself to improving the current treatment options. Using Silastic, he produced a safe and functional shunt, and revolutionized the treatment of children with myelomeningocele [8].
14.2 Associated Abnormalities
The myelomeningocele child will have a level of paralysis based on the position of their spinal cord lesion, but the neurological status is notoriously unstable. These individuals must be monitored carefully to avoid the often preventable neurological deterioration or spinal deformity that may occur due to untreated hydrocephalus, syringomyelia, Arnold–Chiari malformation, or spinal cord tethering (Table 14.3).
Table 14.3
Delayed neurological complications of spina bifida
Seizure disorder |
Hydrocephalus |
± Shunt malfunction |
Arnold–Chiari malformation |
Tethered spinal cord |
Tethered spinal cord with tumor |
Lipoma |
Dermoid |
Neurenteric cyst |
Fibroma |
Diastematomyelia |
Arachnoiditis |
Hydromyelia |
Dermal sinus and stalks |
14.2.1 Arnold–Chiari Malformation
In 1891 and 1896, Chiari described various anatomic patterns of herniation of the brain stem through the foramen magnum [23]. The Chiari II malformation, also known as the Arnold–Chiari malformation, is characterized by the displacement of the medulla oblongata into the cervical canal and an upward course of the cervical nerve roots. Almost all children born with myelomenigocele will have the Chiari II malformation which can cause periodic stridor, apnea, swallowing difficulties, upper extremity paresis, hypertonia, nystagmus, and opisthotonis. The symptoms are worse in early childhood. In most affected children treatment of the secondary hydrocephalus with a ventriculoperitoneal shunt will alleviate the symptoms, but in some a decompression of the posterior fossa will be necessary.
14.2.2 Hydrocephalus
The Arnold–Chiari malformation obstructs the cerebral spinal fluid circulation. At birth, the open communication between the fourth ventricle and the central canal allows for decompression of the cerebrospinal fluid into the myelomeningocele sac. Once the sac is closed, this avenue of decompression is lost and hydrocephalus occurs. A ventriculoperitoneal shunt has been the standard treatment to avoid cortical damage, though recently endoscopic third ventriculostomy alone or in combination with choroid plexus cauterization has shown promise as a better treatment option [98, 99].
Hydrocephalus frequently recurs secondary to shunt malfunction. In children, the clinical signs of acute hydrocephalus are bulging fontanelles, altered mental status, nausea, vomiting, and severe headaches [65]. Hydrosyringomyelia may occur when the cerebral spinal fluid pressure within the central canal increases. Symptoms include increasing lower extremity weakness, spasticity, back pain, rapid progression of scoliosis, and upper extremity weakness. Early correction of hydrocephalus by shunt revision is usually curative.
14.2.3 Tethered Spinal Cord
The spinal cord is considered tethered when the conus medullaris is located at an abnormally distal level and fixed there by an inelastic structure. In the child with myelomeningocele, the spinal cord is tethered not by an expendable and easily resected thickened filum terminale, but by an adherence between the neural placode, the surrounding tissues, and the repaired dural layer. These children may also have dermoid inclusion cysts that add to the tethering or cause pressure on adjacent neural tissue. Most children who have undergone operative closure of a myelomeningocele will demonstrate a low lying spinal cord on magnetic resonance imaging (MRI) examination. In these individuals, the diagnosis of the tethered cord syndrome requires the presence of both anatomic tethering and neurological deterioration, and the absence of other causes of deterioration such as hydromyelia, shunt malfunction, and symptomatic Chiari II malformation. An MRI assessment will define the nature of the tether and assess the presence of possible associated pathologies including diastematomyelia, lipomas, dermoids, granulomas, inclusion cysts, and teratomas [37, 76].
Neurological deterioration due to a tethered cord will occur in up to 30 % or more of children with repaired myelomeningocele [90]. Release should be performed before major functional loss occurs. Although the conus level does not significantly change after detethering [17], the procedure can be expected to produce improvement or stabilization in most cases as illustrated by a comparison of outcomes in two long-term studies of sacral level myelomeningocle populations. In patients that had detethering procedures, none of 62 lost ambulatory ability and 61 were community ambulators [92]. In another similar study of 36 patients that did not have routine untethering, one-third had gait deterioration, 11 became wheelchair-dependent, and spinal deformities and lower extremity contractures developed in 44 % [16]. Results from untethering seem to vary with respect to the symptoms. The results are excellent for improvement of pain and motor deficits [54], but less successful for urological functional [20, 52] and accordingly timely intervention is key.
In properly selected myelomeningocele patients, tethered spinal cord release can stabilize a progressive scoliosis. Pierz et al. evaluated the effect of tethered cord release on scoliosis in 21 myelomingocele patients. Three had improvement in the curvatures, and six stabilized. Twelve patients progressed greater than 10°. Eighty-six percent of patients with initial curvatures over 40° and 100 % of those with a thoracic level required spinal fusion [72]. McLone et al. reported on 30 patients with myelomeningocele and scoliosis. In patients with curvatures greater than 50°, 1 out of 6 improved, while 14 of 23 with less severe curvatures stabilized [53].
14.3 Other Associated Abnormalities
14.3.1 Latex Allergy
Allergy to latex rubber has been reported in up to 28 % of individuals with myelomeningocele [10, 21, 51, 56, 58, 94]. These are IgE-mediated reactions characterized by urticaria, bronchospasm, rhinoconjuntivitis, laryngeal edema, and systemic anaphylaxis. The risk factors for intraoperative anaphylaxis are number of surgeries and atopic predisposition [32, 33, 39, 43, 63]. In the past, an attempt was made to identify latex-sensitive patients, but this strategy proved inefficient. A negative history for reaction does not eliminate the possibility of intraoperative anaphylaxis; skin testing lacks sensitivity and safety; and preoperative prophylaxis is not dependable. Accordingly, the present recommendation is that all myelomeningocele patients be treated in a latex-free environment.
14.3.2 Precocious Puberty
Precocious puberty in girls with myelomeningocele is common. Proos et al. noted breast development by age 9 and related the risk of precocious puberty to increased intracranial pressure and shunt malfunction [74]. Furman and Mortimer noted that affected girls began menstruation at an average age of 10 years 3 months and earlier than their mothers and siblings [28].
14.3.3 Spinal Deformity
Spinal deformity occurs commonly and early in children with myelomeningocele. Piggott noted a 90 % incidence of some form of spinal deformity by age 10 years, and in half the patients the curvature was significant enough to merit surgical treatment [73]. The common deformities are scoliosis and hyperkyphosis which are often classified as congenital and developmental. Congenital deformities include scoliosis and kyphosis due to failure of formation or segmentation, and the rather unique form of kyphosis found in the myelomeningocele population due to dysplastic posterior elements. Developmental curvatures occur without vertebral malformation presumably due to muscle paralysis, muscle malposition, or hip deformity and pelvic obliquity [73, 75]. More recently, hydrocephalus, syringomyelia, and spinal cord tethering have been appreciated as causative factors. Spondylolisthesis has also been reported [42, 88].
Problems caused by spinal deformity include recalcitrant ulcerations over the gibbus in individuals with kyphosis or of the ischium or sacrum in wheelchair sitters with scoliosis and trunk imbalance. Pulmonary compromise can occur with significant scoliosis or kyphosis. Those with trunk imbalance in either the sagittal or frontal plane may need to use their hands for support and in doing so lose upper extremity function [6, 38, 41, 81]. Scoliosis is the most common deformity and will be discussed. Kyphosis is covered elsewhere in this text.
14.3.3.1 Scoliosis
Scoliosis of 10° or more will occur in 50–80 % of myelomeningocele children by 10 years of age [59, 73, 84]. Congenital scoliosis is present in 7–38 % of these [75, 80]. This will progress when spinal growth is unbalanced. Treatment is similar to that of congenital scoliosis not associated with spinal dysraphism, but in the myelomeningocele group posterior fusion alone in the dysplastic portion of the spine is likely to fail due to the deficient bone stock. The addition of anterior fusion has been recommended [46].
Developmental scoliosis is the more common variety, and its incidence relates to the level of the neurological and anatomical lesion. Trivedi et al. defined scoliosis in this group as a curvature greater than 20° and noted the three most predictive factors for the development of scoliosis to be motor level, ambulatory status, and last intact laminar arch (LILA). The scoliosis prevalence was 93 %, 72 %, 43 %, and 7 % in patients with thoracic, upper lumbar, lower lumbar, and sacral motor levels respectively, and 89 %, 44 %, 12 %, and 0 % at similar LILA, respectively [95]. A rate of progression during growth of 12.5° per year in curvatures over 40° can be expected with a maximum rate in the 11–15-year-age group [61].
14.3.3.2 Orthotic Management
While many believe orthotic treatment to be at best temporizing [27, 34, 45, 65, 77], Muller and Nordwall [60] reported that the Boston brace arrested progression if initiated before the curvature reached 45°. Whether or not it prevents progression, an orthosis can improve function by providing improved support for seating. The rigid “active” control braces utilized in idiopathic deformities are problematic in the neuromuscular population, but total contact “soft” orthoses seem to be well tolerated. Letts et al. reported on the use of the “soft Boston orthosis” in the management of neuromuscular scoliosis. The brace is constructed of “Aliplast,” a material similar to Styrofoam, and reinforced with polyethylene. While the improvement of the scoliosis averaged 15°, postural position or seating stability was enhanced 90 % [44]. Orthoses may be modified to accommodate individual needs (Fig. 14.2).
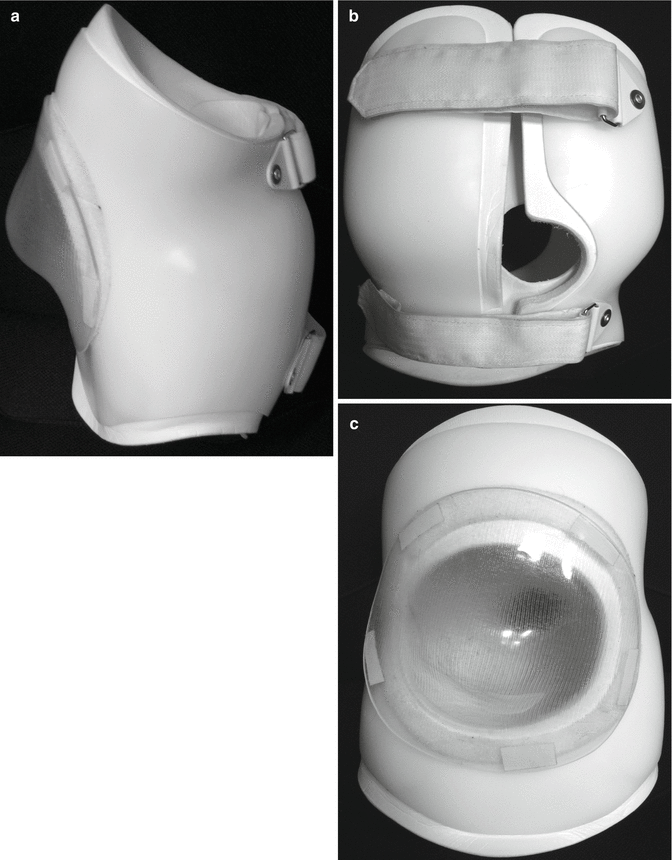

Fig. 14.2
Spinal orthoses may not prevent deformity progression, but may improve function. Here a “soft” brace has been reinforced with polypropylene and adapted to accommodate a rigid kyphotic deformity with skin ulceration (a–c). The removable clear plastic bubble over the gibbus protects the skin while allowing for its easy inspection. The orthosis is further modified to allow for drainage catheters. The device allowed this child with skin ulceration over the gibbus to maintain full function during treatment
14.3.3.3 Surgical Indications and Planning
Surgery is frequently suggested for curvatures over 50° [9, 65] presumably because a deformity of this magnitude will be problematic or will progress to the point of being so. There is little evidence-based data to support either premise [103]. There are no long-term studies that document either the progression or clinical consequences of curvatures in adulthood. Improved sitting balance is an immediate benefit of scoliosis surgery but is obtained with considerable risk. Skin ulceration due to seating imbalance is another indication for surgical correction of deformity, yet the problem can be exacerbated following surgery if rigid residual pelvic obliquity persists [24, 66] or lordosis is diminished [52].
There are arguments that can be made for and against surgical treatment and discussions with families need to be balanced. Surgeons that believe that the natural history of untreated scoliosis is sufficiently problematic to require surgery should temper their discussions with reference to the paucity of evidence-based studies and the very real risks of complications and functional loss.
Preoperative Evaluation
This is a multisystem disease that requires an extensive preoperative evaluation. The skin in the area of the surgery must be assessed for scarring and fragility as large corrections and bulky spinal instrumentation can make closure difficult. Tissue expanders used preoperatively may be helpful in allowing better soft tissue coverage, though reported experience is limited [35, 68].
The gait and method of active transfers should be observed to determine the consequences of the loss of lumbosacral motion following extensive fusions. Hip contractures may become more problematic when the lumbosacral spine is fused. Diminished hip extension will be further compromised if the lordosis is surgically reduced and stance and ambulation will become more difficult. Increased lordosis following fusion will make sitting more difficult in those with reduced hip flexion at baseline.
A thorough neurological examination is performed. The Arnold–Chiari malformation, hydrocephalus, or spinal cord tethering may be the cause of the progressive scoliosis and their treatment may preclude the need for the surgery. If symptomatic and untreated, they may complicate the procedure. Shunt function must be evaluated prior to cordotomy or intraoperative spinal cord manipulation to avoid catastrophic complications [30, 102]. An MRI should be performed to evaluate for syringomylia and intraspinal tumors as well as spinal cord tethering. The conus will essentially always be further distal than the norm, and this finding must be put in context with other findings to determine if there is symptomatic tethering. The role of prophylactic detethering prior to surgery is not established [79].
Laboratory assessment must include a urine culture and nutritional evaluation. Hatlen et al. have demonstrated that nutritional deficiency and preoperative positive urine cultures were related to an increased risk of infection. The organism responsible for the deep wound infection was the one found in the preoperative urine culture in 66 % of the cases [36].
The radiographic assessment should include full-length views of the spine in the position of function: standing for ambulators and sitting for non-ambulators. A sitting upright will eliminate the affect of hip contractures on the spinal alignment. This view when performed anterior/posterior as opposed to the posterior/anterior fashion affords better assessment of the lumbosacral articulation and pelvic obliquity. Flexibility is assessed with side bends, traction, or fulcrum bends as needed to determine the need for releases or other destabilizing measures and the extent of the fusion. Maturity is best evaluated by bone age as chronological age is less accurate in a population with frequent precocious puberty. A spinal CT scan is helpful to better analyze the three-dimensional anatomy. It is especially useful in determining the size and orientation of the pedicles in the dysplastic portion of the spine when pedicle fixation is planned (Fig. 14.3).
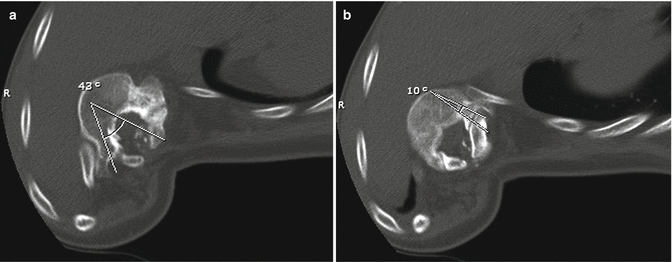

Fig. 14.3
While pedicle screws offer superior fixation in the dysplasic portion of the spine, placement can be challenging. The pedicle orientation and size may vary significantly within (a, b) and between levels. The vertebral body depicted is at the apex of the deformity where the posterior elements are essentially intact, but the angles for pedicle screw insertion and allowable screw length differ significantly
Surgery
The surgical treatment of spinal deformity in children with myelomeningocele is arguably the most challenging type of spinal surgery. The deformities are often long, severe, and rigid. There is poor soft tissue coverage and the skin is often insensate, scarred, and fragile. The posterior elements are deficient and dysplastic, offering a poor mass for fusion and difficult instrumentation purchase and placement. There may be tenuous neurological status and associated urological abnormalities.
The timing of surgery is another matter for consideration. Children that develop scoliosis will usually do so before age 10 years. The decision will be to use either growth preserving techniques or definitive surgery. One consequence of early fusion will be shortened trunk length that translates into less lung volume and perhaps lung growth. The crankshaft phenomenon has been noted in immature patients with scoliosis that have undergone posterior fusion. The continued anterior growth and posterior tether results in a rotational deformity. This deformity has not been documented in the myelomeningocele population and there is some evidence that posterior instrumentation to the pelvis in the neuromuscular population protects against it [86, 100]. The benefits of additional trunk growth must be weighed against the risk of repeated surgeries in a population notoriously at risk for operative complications.
Definitive Surgery
Advancements in technique have had a significant impact on the results of deformity surgery. Combined anterior and posterior surgery has lowered the rates of nonunion. Segmental instrumentation allows for secure fixation through the dysplastic posterior elements, dramatic corrections, and reduction or elimination of postoperative immobilization.
The choice of surgical methods must be individualized based on the patient’s needs and function, the vertebral deficiency, and deformity characteristics. Attempts to spare mobile lumbar segments seem appropriate in ambulatory patients. More rigid and severe curvatures with extensive dysplastic segments will require more secure posterior fixation obtained through increased segmental anchors and, usually, the inclusion of the pelvis. The stresses on those fixation anchors can be lessened through anterior releases or posterior destabilization techniques.
Combined Anterior Posterior Instrumentation and Fusion
In the myelomeningocele population, combined anterior and posterior spinal procedures have been the standard of care. In his evidence-based literature review, Wright demonstrated that the best available series were consistent Level II and III studies. These studies clearly demonstrated the superiority of fusion rates and correction in the combined anterior and posterior techniques (Table 14.4).
Table 14.4
Myelomeningocle scoliosis surgical correction: a comparison of instrumentation techniques
Author | Year | Scoliosis correction (%) | Pelvic obliquity (%) | Infection | Pseudo/instrument failure | |
|---|---|---|---|---|---|---|
Post non-segmental | Osebold [67] | 1982 | 23 | 29 | 33 % | 46 % |
Mazur [52] | 1986 | 32 | 39 | 7 % | 33 % | |
Parisini [69] | 2002 | 17 | 50 | 30 % | 70 % | |
Post non-segmantal and anterior fusion | Ward [97] | 1989 | 57 | 60 | ||
Osebold [67] | 1982 | 48 | 46 | 50 % | 100 % | |
Post non-segmental and anterior instrumentation | Osebold [67] | 1982 | 56 | 47 | 18 % | 23 % |
DeWald [22] | 1979 | 55 | ||||
Mayfield [50] | 1981 | 75 | 0 | 31 % | ||
Mazur [52] | 1986 | 42 | 41 | 11 % | 11 % | |
McMaster [55] | 1987 | 63 | 72 | |||
Anterior only | Mazur [52] | 1986 | 48 | 14 | 29 % | |
Sponseller [87] | 1999 | 57 | 44 | 7 % (superficial) | 36 % revision | |
Basobas [12] | 2003 | 59 | 64.3 | 5 % | 24 % revision | |
Post-segmental and anterior fusion | Ward [97] | 1989 | 51 | 56 | ||
Banta [11] | 1989 | 53 | 11 % | 16 % | ||
Parsch [70] | 37 | 33 % | ||||
Geiger [30] | 1999 | 52 | 33 % | |||
Yazici [104] | 2000 | 68 | 96 | |||
Post-segmental and anterior instrumentation | Ward [97] | 1989 | 63 | 71 | 18 % | |
Geiger [30] | 1999 | 59 | ||||
Parsch [70] | 2001 | 57 | 18 % | |||
Wild [101] | 2001 | 56 | 81 | 27 % (superficial)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





