1
Six or more café-au-lait macules more than 5 mm in greatest diameter in prepubertal individuals and more than 15 mm in postpubertal individuals
2
Two or more neurofibromas of any type or more than one plexiform neurofibroma
3
Freckling in the axillary or inguinal regions
4
Two or more Lisch nodules (iris hamartomas)
5
Optic glioma
6
A distinctive osseous lesion, such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
7
A first degree relative (parent, sibling, or offspring) with NF-1 by the above criteria
16.2.2 Neurofibromatosis 2 (NF-2)
NF-2 or central neurofibromatosis has an estimated incidence of 1 in 33,000 individuals and is associated with bilateral vestibular schwannomas and multiple spinal shwannomas [23, 24]. The NF-2 locus is located on the long arm of chromosome 22. Fifty percent of cases involve a new mutation. NF-2 is not associated with primary skeletal disorders; however, multiple paraspinal and intraspinal tumors (schwannomas and ependymomas) are common in this disorder. NF-1 and NF-2 are genetically distinct disorders with different gene loci, despite similarities in names.
16.2.3 Segmental Neurofibromatosis
Segmental neurofibromatosis is characterized by features of NF-1 involving a single body segment. Typically, only a single segment of the body (such as left upper extremity) is affected with café-au-lait spots and freckling, and lesions usually do not cross the body midline. Other segmental forms may involve deep neurofibromas in a single body segment. It is considered as a somatic mosaic form of NF-1, and typically is not associated with cognitive effects or learning disabilities seen in NF-1.
16.2.4 Legius Syndrome
Early neurofibromatosis literature recognized that a mild form of NF-1 existed, consisting primarily of familial café-au-lait spots. In recent years, multiple families with such mild involvement have now been found to have mutations in the SPRED1 gene. Initially discovered by Legius et al. [25] this condition, now called Legius syndrome, can present with multiple café-au-lait spots, freckling, macrocephaly, and mild learning disabilities, but does not present with any of the benign or malignant tumors associated with NF-1. This condition is quite a bit less common than NF-1, with an estimated prevalence of about 1/50,000. Since patients with Legius syndrome can actually meet the clinical diagnostic criteria for NF-1, it can be appropriate to perform molecular testing if there is any question about diagnosis.
16.2.5 Schwannomatosis
Schwannomatosis is a distinct form of neurofibromatosis which typically involves multiple schwannomas throughout the body, but without the vestibular schwannomas typical of NF-2. Initially thought to represent a mosaic form of NF-2, it has now been determined that familial schwannomatosis is due to mutations in the INI1 gene, linked to NF–2 on chromosome 22. It is a disease of adulthood that consists of multiple deep painful peripheral nerve sheath tumors that may occur in a generalized form or in a segmental distribution. Differential diagnosis from NF-2 can be difficult, and genetic testing of NF-2 and INI1 is now available to help in making this distinction.
16.3 Spinal Abnormalities in NF-1
16.3.1 Epidemiology
Spinal abnormalities are the most common orthopedic manifestation of NF-1. It is quoted as from 2 to 36 % in the literature [10, 11]. In the NF clinic at our institution, it is 23 % [12]. In a report in 1988, Winter et al. [26]. found only 102 patients having NF-1 by clinical criteria in a pool of approximately 10,000 patients with scoliosis. Functional scoliosis resulting from limb hypertrophy or long-bone dysplasia leading to limb length inequality must be ruled out in patients with NF-1. Rarely, unrecognized extra-pleural thoracic tumors can present as focal scoliosis. These lesions are usually plexiform neurofibroma and are not visible on plain radiographs [27]. The spinal deformities tend to develop early in the life therefore, all preadolescent children with NF-1 should be evaluated by scoliosis screening or the Adam forward-bend test to rule out the presence of a spinal deformity.
It is important to emphasize that there is no standard pattern of spinal deformity in NF-1. All manner of spinal deformities in multiple planes and in any part of the spine may occur with NF-1 [28, 29]. The characteristic deformity tends to be a short-segmented, sharply angulated curvature that usually involves four to six vertebrae in the upper third of the thoracic spine [30]. We have traditionally classified the deformities into dystrophic or non-dystrophic types based on the coronal plane x-rays. The entire spine may be affected by deformity in the coronal and sagittal planes. There are nine radiographic criteria most often used to classify the deformity as dystrophic. These include rib penciling (the rib being smaller in diameter than the second rib), vertebral rotation, posterior vertebral scalloping, vertebral wedging, spindling of the transverse process, anterior vertebral scalloping, widened interpedicular distance, enlarged interverteral foramina, and lateral vertebral scalloping. Recently, two more magnetic resonance imaging (MRI) findings have been added to the criteria used to classify the deformity as dystrophic: the presence of dural ectasia and the presence of paraspinal tumors (Table 16.2) [31]. More than three of these dystrophic features are considered diagnostic of dystrophic scoliosis. Non-dystrophic curves are considered similar to idiopathic scoliosis.
Table 16.2
Diagnostic criteria of dystrophic spine
1 | Rib penciling |
2 | Posterior vertebral scalloping |
3 | Vertebral wedging |
4 | Spindling of transverse processes |
5 | Anterior vertebral scalloping |
6 | Widened interpedicular distance |
7 | Enlarged intervertebral foramina |
8 | Lateral vertebral scalloping |
9 | Vertebral rotation |
10 | Paraspinal tumors |
11 | Dural ectasia |
16.3.2 Etiology
The cause of spinal deformity remains unknown. Several theories including metabolic bone deficiency, osteomalacia, endocrine disturbance, and mesodermal dysplasia have been proposed and are at best inconclusive [32–36]. The dystrophic changes may be attributed to intrinsic factors or may be associated with anomalies of the spinal canal secondary to abnormalities of the spinal cord dura mater.
Pressure erosive effects of dural ectasia and paravertebral tumors have been frequently found to be adjacent to and approximated to the deformities, initiating instability and subsequent deformity. Dural ectasia, a disorder unique to certain conditions, is an expansion or dilatation of the dural sac. The changes in the spinal canal induced by dural ectasia may increase the difficulty in obtaining adequate purchase for fixation of anchors during spinal deformity correction.
Scalloping was initially thought to represent the result of erosive pressure or direct infiltration of the vertebra by adjacent neurofibroma [37–41]. A neurofibroma-derived locally active biochemical substance or hormone that triggers dystrophic features in the adjacent vertebra has also been proposed [37]. The presence of an altered response of the vertebral bone in NF-1 to a paraspinal tumor has been hypothesized. An interactive pathophysiological mechanism between a genetically compromised bone and a neuroectodermal derivative, such as a contiguous neurofibroma or an abnormal meningeal sheath, is suggested by some authors [37, 39].
The etiological theory of vertebral scalloping being a primary developmental defect was supported by the presence of scalloping without adjacent lesions [42]. This was also supported by an MRI study in patients with NF-1, in which posterior vertebral scalloping was highly associated with dural ectasia, lateral scalloping was related to dural ectasia or neurofibromas in 50 % of cases, and anterior scalloping was unrelated to dural ectasia or tumors [43]. The authors could not identify any association with dural ectasia or paraspinal tumors in more than one-third of their patients with MRI evidence of vertebral scalloping. Nevertheless, dural ectasia without associated vertebral scalloping was recorded in 10 % of the cases.
A recent study in ten monozygotic twins with NF-1 demonstrated mixed concordance and discordance for presence of scoliosis [3]. The affected twin pairs were discordant for presence of dystrophic features, degree of curvature, and need for surgery. This finding suggests that both heritable and nonheritable factors contribute to the pathogenesis of spinal deformities in NF-1 patients. Dystrophic curves most likely require a nonhereditary event, such as an adjacent tumor or dural ectasia, or a second hit event in local bone cells leading to the underlying dysplasia. If occurrence and progression of dystrophic spinal deformity is affected by adjacent neurofibromas, then therapies targeting to reduction or stabilization of paraspinal tumors could provide a promising approach to spine deformity prevention in patients with NF-1.
Apart from its tumor suppressor activities through the Ras signaling, the role of neurofibromin may converge with other bone biochemical pathways, such as bone morphogenetic protein (BMP) signal transduction [44]. This theory suggests that intrinsic bone pathology due to loss of a functional NF–1 allele with subsequent Ras deregulation may be responsible for osseous manifestations in NF-1 through altered osteoblastic/osteoprogenitor differentiation, overgrowth of cellular tissue due to preferred fibroblast differentiation of mesenchymal cells, and impaired bony callus formation. Double inactivation of NF-1 by somatic mutation of the NF–1 gene in a population of cells which depends on neurofibromin-regulated Ras signaling to maintain normal bone was suggested to contribute to the occurrence or progression of tibia pseudarthrosis [45]. Although such second hit events have been demonstrated in pathological tissue from NF-1 tibias, it is unknown whether spinal deformities of NF-1 require a second hit event.
16.3.3 Mouse Models
The NF-1 heterozygous mouse has a minimal skeletal phenotype. In order to better understand the mechanism of skeletal abnormalities in NF-1, researchers have developed more complex mouse models with knock-out of the second NF–1 allele in osteoprogenitor cell lines, using a process called cre-recombination. In one such model, Col2.3Cre(+) mice showed multiple vertebral anomalies, including: short vertebral segments, reduction in cortical and trabecular bone mass of the vertebrae, increased numbers of osteoclasts, and decreased numbers of osteoblasts in vertebrae [45]. These mouse models provide additional insight to the underlying pathophysiology of spinal abnormalities in humans with NF-1.
16.3.4 Imaging
Most often plain standing posterior–anterior and lateral radiographs are sufficient for screening the curvature. An angle of greater than 10° assigns the deformity as structural. When treatment is to be initiated, multiple planar films in supine bending modes and traction are necessary to determine flexibility. If there are adjacent structures requiring further clarification, higher levels of imaging are required, such as computed tomography (CT) for bony deformity or high-resolution contrast CT or MRI for soft tissue delineation.
16.3.5 Dural Ectasia
Dural ectasia is a circumferential dilatation of the dural sac which is filled with proteinaceous fluid. The slow expansion of the dura results in erosion of the surrounding osseous structures resulting in widening of the spinal canal, thinning of the laminae, and ultimately destabilization of the spine. Dural expansion through the neural foramina can cause meningoceles giving the radiographic dumbbell appearance. However, enlargement of a single neural foramen on an oblique radiograph is usually caused by neurofibroma exiting from the spinal canal rather than from the dural ectasia (Fig. 16.1). Similar lesions are seen in other connective tissue disorders, e.g., Marfan’s syndrome and Ehler–Danlos syndrome, although cause of these lesions in NF-1 is not known.
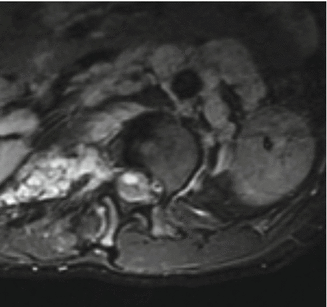

Fig. 16.1
MRI of the spine with the neurofibroma in canal. Bright shadow depicts neurofibroma exiting through the spinal canal. The constriction of the neurofibroma in the foramen gives it the appearance of a dumbbell
During this process, the neural elements are not affected. As a result of slow nature of this process and enormous widening of the spinal canal the neural elements have adequate room for accommodation, and there may be severe angular deformity and distortion without neurological deficit. The patients remain neurologically intact until later in the course of the disease process when destabilization of the vertebral column jeopardizes the neural elements. Dislocation of the vertebral column due to dural ectasia has been reported in the literature [46]. The destabilization at the costovertebral junction can result in penetration of the rib head into the spinal canal with neurological compromise (Fig. 16.2) [47, 48]. The presence of rib head or the neurofibroma in the spinal canal can result in intraoperative neurological deficit if instrumentation is used for correction of the curve without adequate decompression.

Fig. 16.2
Dislocation of rib head in the spinal canal in a severe dystrophic thoracic curve. Careful evaluation of the preoperative imaging including CT scan is essential to identify this pathology. Decompression prior to correction is essential to prevent intraoperative neurological complications
Dural ectasia can be readily seen on high-volume CT myelography or contrast-enhanced MRI and is recommended before surgical intervention is undertaken for dystrophic curves. Higher imaging studies help to demonstrate extremely thin laminae; in which case dissection by electocautery rather than by periosteal elevators are recommended during surgical exposure to avoid direct injury to the neural elements/dura by plunging into the spinal canal. Surgical spinal stabilization and fusion does not alter the course of dural ectasia. Dural ectasia can result in failure of the primary fusion or the expanding dura ultimately can destroy a solid fusion leaving behind the instrumentation (Fig. 16.3).
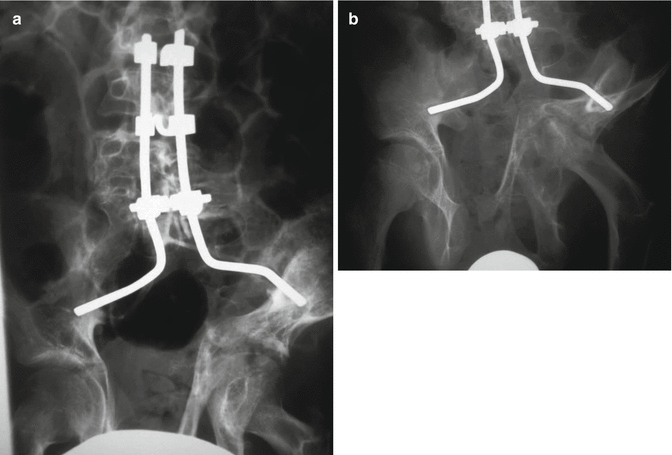

Fig. 16.3
(a) Neurofibromatosis in the lumbosacral spine. This patient was treated by fusion and instrumentation extending to the pelvis. (b) A few years later, the fusion mass and the vertebrae are eroded completely by expanding dural ectasia leaving behind the instrumentation. Note also the destruction of the hip joint by tumor
Spinal affections in NF-1 can be described under following regions: cervical, thoracic/thoracolumbar, lumbosacral, and spinal canal.
16.4 Cervical Spine Abnormalities
The cervical spine abnormalities in NF-1 have not received enough attention in the literature [49, 50]. Usually, the cervical lesion is asymptomatic. When the lesion is symptomatic, pain is the most common presenting symptom [51]. Cervical abnormalities are likely to be missed in presence of scoliosis or kyphoscoliosis lower regions of the spine where the examiner’s attention is focused on the more obvious deformity. In a study of 56 patients with NF-1, Yong-Hing et al. [52] reported that 17 patients (30 %) had cervical spine abnormalities. Out of these, seven patients were asymptomatic, whereas the rest had limited motion or pain in the neck. Four patients had neurological deficits that were attributed to cervical instability. Four of the 17 patients required fusion of the cervical spine. Curtis et al. [53] described eight patients who had paraplegia and NF-1. Four of these patients had cervical spine instability or intraspinal pathology in the cervical spine. The upper cervical spine should also be examined carefully. Isu et al. [54] described three patients with NF-1 who had C1–C2 dislocation with neurological deficit. All patients improved after decompression and fusion. We recommend that the cervical spine should be evaluated at the initial scoliosis assessment.
A lateral radiograph of the cervical spine is the initial screening tool. The NF-1 can be manifested on a plain radiograph in the form of dystrophic changes or malalignment [55]. If any suspicious area is noted on plain radiographs, right and left oblique views should be obtained to look for widening of the neuroforamina which may represent dumbbell lesions. MRI is the definitive study to evaluate these lesions.
Anteroposterior and lateral radiographs of the cervical spine should be obtained in all NF-1 patients who: (1) are placed in halo traction; (2) undergo surgery; (3) require endotracheal intubation; (4) present with neck tumors; (5) complain of neck pain; and (6) present with symptoms indicating intra- or extraspinal neurofibromas, such as torticollis or dysphagia [56]. If there is any suspicion of instability, CT and/or flexion-extension MRI are indicated. Erosive defects of the skull may be present in some patients with NF-1. Thus, plain radiographs of the skull prior to halo or Gardner–Wells tong traction pins application are strongly recommended.
The most common spinal abnormality in the cervical spine is a severe cervical kyphosis (Fig. 16.4), which is often seen following a decompressive laminectomy without stabilization for an intraspinal lesion and is highly suggestive of the disorder [57]. We recommend stabilization of the spinal column at the same time of surgical removal of tumors from the spinal canal.
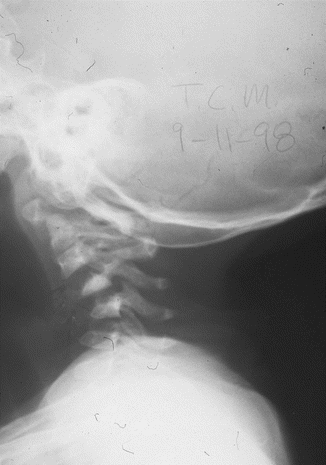

Fig. 16.4
Lateral radiograph of the cervical spine 6 months following laminectomy and excision of the neurofibroma, demonstrating marked kyphosis of the entire cervical spine. Note the dystrophic appearance of the vertebrae
Ogilvie reported on the surgical treatment of cervical kyphosis by anterior fusion with iliac-crest or fibular bone graft or both [51]. He considered halo traction to be a useful preoperative step if the kyphosis was greater than 45°. In the presence of progressive cervical kyphosis, we recommend preoperative halo traction only if the deformity is flexible as judged by the radiographs. This should be followed by posterior fusion. If the deformity is rigid, then an anterior soft-tissue release followed by traction is safer.
Internal fixation with pedicle and lateral mass screws is preferred for posterior instrumentation. Sublaminar wire fixation may be difficult secondary to dural ectasia and osseous fragility. For anterior fixation, we currently use bioabsorbable plates. Even with rigid instrumentation, postoperative halo immobilization is recommended until a fusion mass with trabecular pattern is seen on cervical CT.
16.5 Thoracic/Thoracolumbar Spinal Abnormalities
The two varieties of spinal deformity are well distinguished in these regions of the spine. Also, the natural history of spinal deformities is well studied for thoracic/thoracolumbar region.
Patients more likely to develop progressive scoliosis of the thoracolumbar spine are children under 7 years of age who have thoracic lordosis (sagittal plane angle of less than 20° measured from T3 to T12) and paravertebral tumors. There is a strong association between modulation and progression of the spinal deformity. More specifically, curves that acquire either three or more penciled ribs or a combination of any three dystrophic features will almost certainly progress [28]. Other factors that have been associated with substantial curve progression include: (1) high Cobb angle at presentation; (2) early age of onset; (3) abnormal kyphosis; (4) vertebral scalloping; (5) severe apical rotation; (6) location of the apex in the middle-lower thoracic spine; (7) penciling of one rib or more on the concave side or both sides of the curve; and (8) penciling of four ribs or more [34].
More recent MRI studies have questioned the theory of modulation [43]. Patients with radiographically labeled non-dystrophic curves have been found to have significant dysplastic changes on MRI. Having in mind the higher sensitivity of MRI in identification of dystrophic features than x-rays, we recommend characterization of the curve as dystrophic or not based on a combination of MRI and x-ray findings [31].
16.5.1 Non-dystrophic Scoliosis
This is the common variety of spinal deformity observed in NF-1. These curves behave similar to idiopathic curves with some differences [7, 9, 58]. This form usually involves 8–10 spinal segments. Most often, the deformity is convex to the right. However, these curves usually present earlier than the idiopathic curves and are more prone to progression. Furthermore, the rate of pseudoarthrosis following a fusion surgery is higher in these patients [49]. These differences can be attributed to the process of modulation and the underlying bone pathology. Compared to dystrophic curves, non-dystrophic curves tend to present in older children with less angulation and rotation of the deformity [59].
16.5.2 Dystrophic Scoliosis
This is an uncommon but malignant form of spinal deformity. It is characterized by early onset, rapid progression and is more difficult to treat [60, 61]. Typically, the dystrophic curve is a short-segmented, sharply angulated type that includes fewer than six spinal segments. Dystrophic curves may be associated with kyphosis and have a higher incidence of neurological injury [61, 62].
Dystrophic vertebral changes develop over time (Table 16.2). Dystrophic curves are found most commonly in the thoracic region (Figs. 16.5, 16.6, 16.7, 16.8, 16.9, and 16.10) [63].
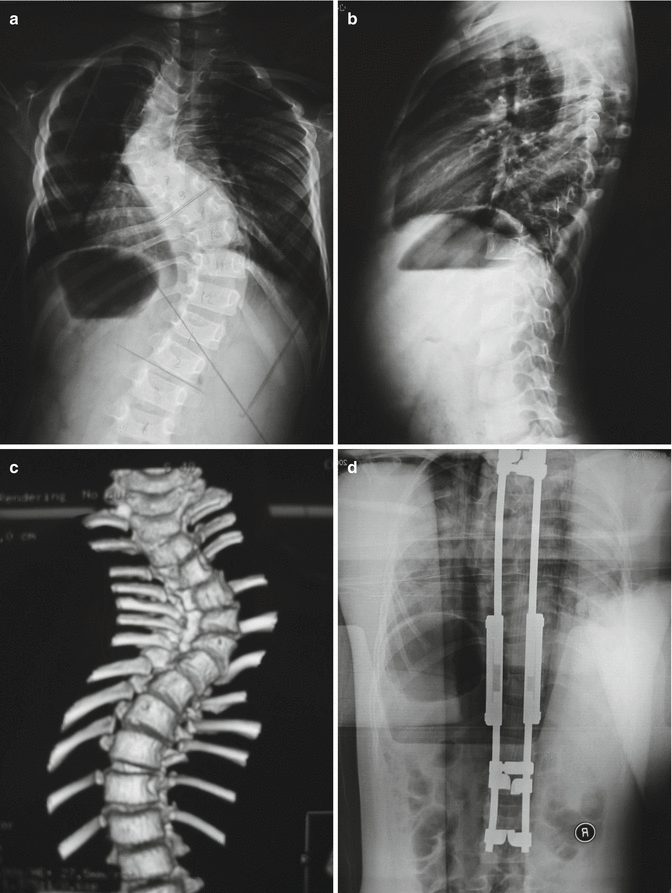
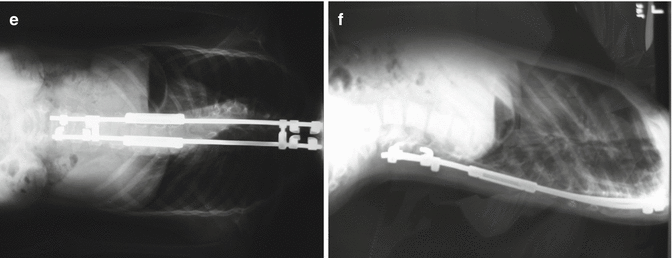
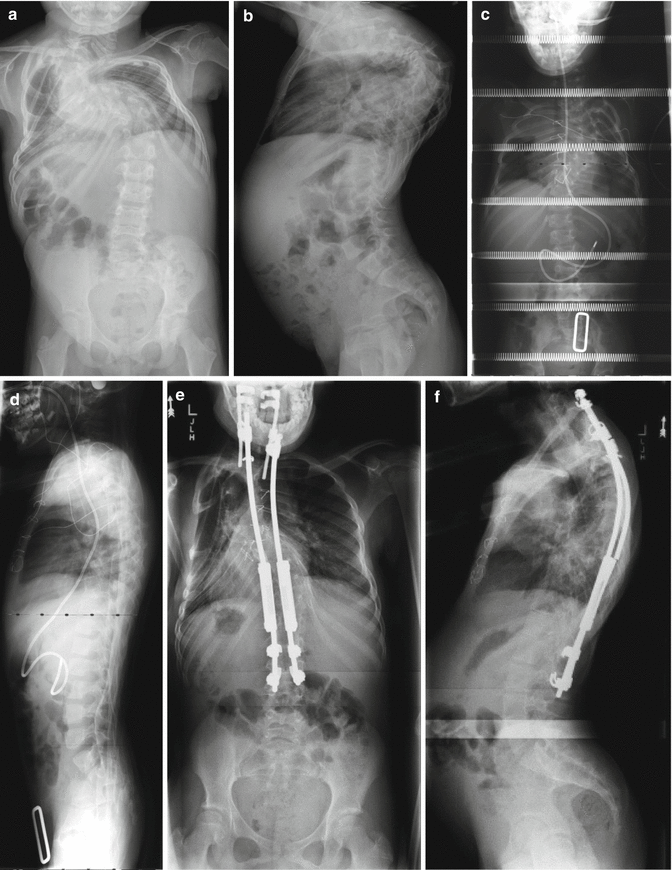
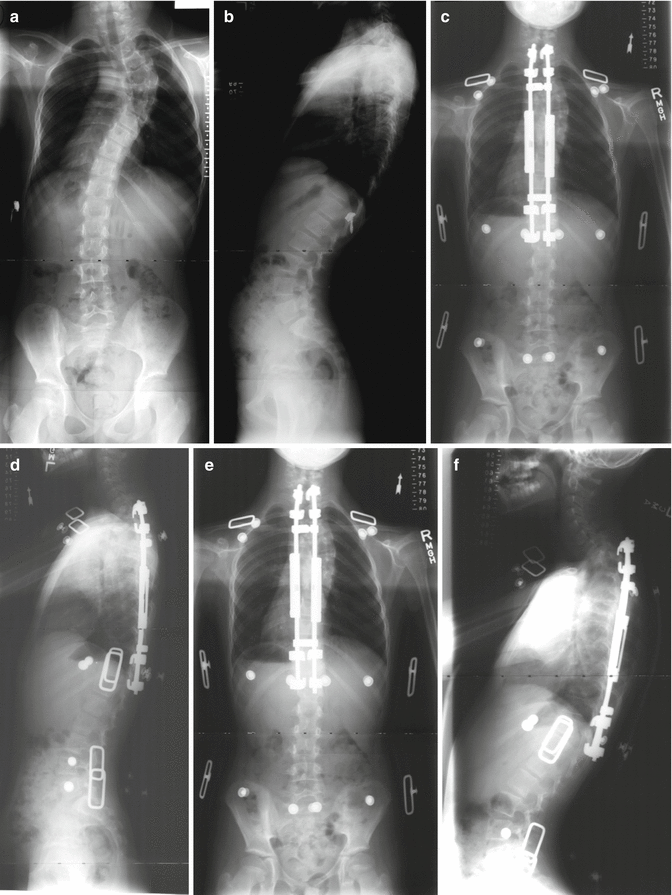
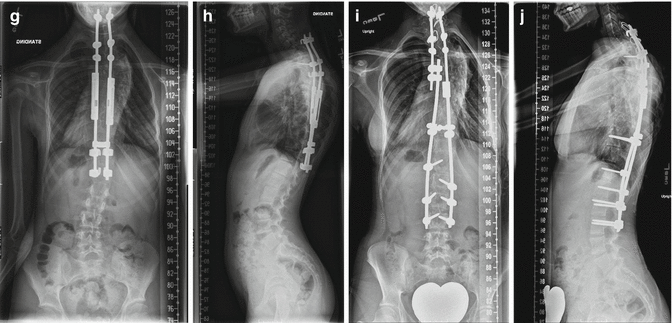
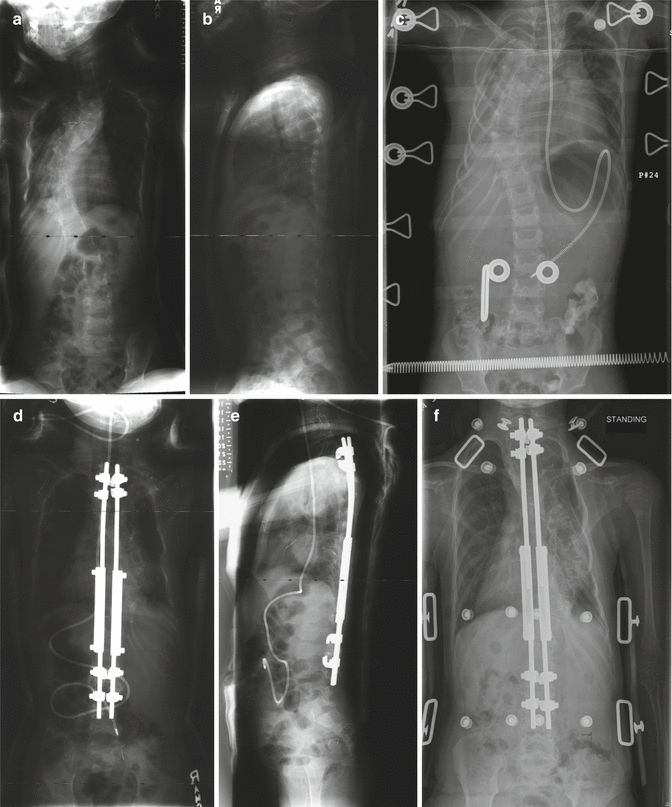
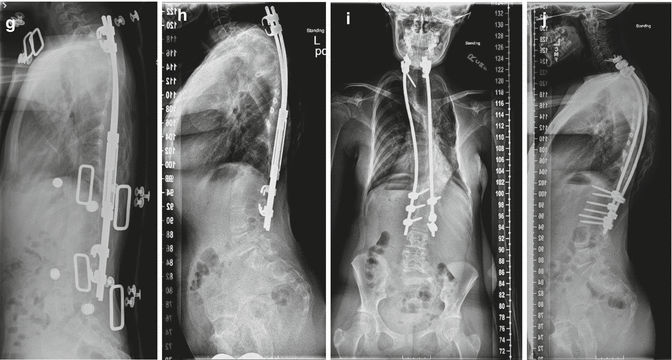


Fig. 16.5
A 6-year-old female with 80° thoracic scoliosis which was untreated. Preoperative 3D CT scan and x-rays (a–c) show presence of typical radiological features of dystrophic vertebral bodies. She underwent a growing rod instrumentation with hook anchors (d). At 2-year follow-up, the correction has remained stable and spinal length has increased following serial lengthenings at every 6-month intervals (e, f)

Fig. 16.6
(a, b) A 6-year-old female with severe thoracic dystrophic kyphoscoliosis. The deformity involves mid and upper thoracic spines. The curvature measures more than 100° in both planes. (c, d) She underwent an anterior release (annulotomies) through a double “trap door” approach for her upper thoracic and mid thoracic curve followed by a period of 2 weeks of halo-femoral traction. Anterior release with gradual traction alone resulted in significant correction of the deformity. (e, f) After the traction, the patient underwent growing rod instrumentation with proximal anchors in her lower cervical spine. At 5 years of follow-up, although one rod is broken, her correction is well maintained and her spinal height has increased as measured by digital radiographs


Fig. 16.7
(a, b) A 7-year-old female with high thoracic dystrophic scoliosis. The brace is usually ineffective in controlling the high thoracic curves. (c, d) The patient underwent a growing rod instrumentation with brace. Decent correction of the curve was achieved with the index procedure. Note that the proximal hook is at T1. (e, f) After 1-year postoperation, the correction is well maintained after two lengthenings. On the lateral x-ray, gradual development of junctional kyphosis is evident at both proximal and distal end instrumented segments. Patient is asymptomatic at this point in time. (g, h) The proximal instrumentation was extended to C7 with supralaminar hooks, which pulled out 2 years after surgery. The instrumentation was then extended to C5 with fusion. (i, j) Final fusion was performed 5 years after the index procedure


Fig. 16.8




(a, b) A 4-year-old female with thoracic dystrophic scoliosis who failed cast-brace treatment. (c–e) She underwent anterior annulotomies at the thoracic apex by thoracoscopic procedure followed by traction for 10 days. This was followed by growing rod instrumentation. (f, g) At 2-year follow-up, the correction has remained stable and spinal length has increased following two lenghtenings. Development of proximal junctional kyphosis at this point in time is asymptomatic. (h) Continued junctional kyphosis lead to prominent hooks. (i, j) Eight years after the index procedure, the patient had menses and she underwent final fusion with exchange of all instrumentation (4.5–5.5 system with transitional rod) with proximal extension to C6. Proximal junctional kyphosis has been corrected satisfactorily. The thoracolumbar spine was solidly fused due to prolonged immobilization by the growing rods and did not require any further anchors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








